Preparation method of lithium iron borate
A technology of lithium iron borate and iron nitrate, applied in borates, chemical instruments and methods, boron compounds, etc., can solve problems such as easy absorption of moisture and oxygen, large specific surface area of products, and decline in product performance, and achieve capacity decay Low efficiency, good stability and small particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
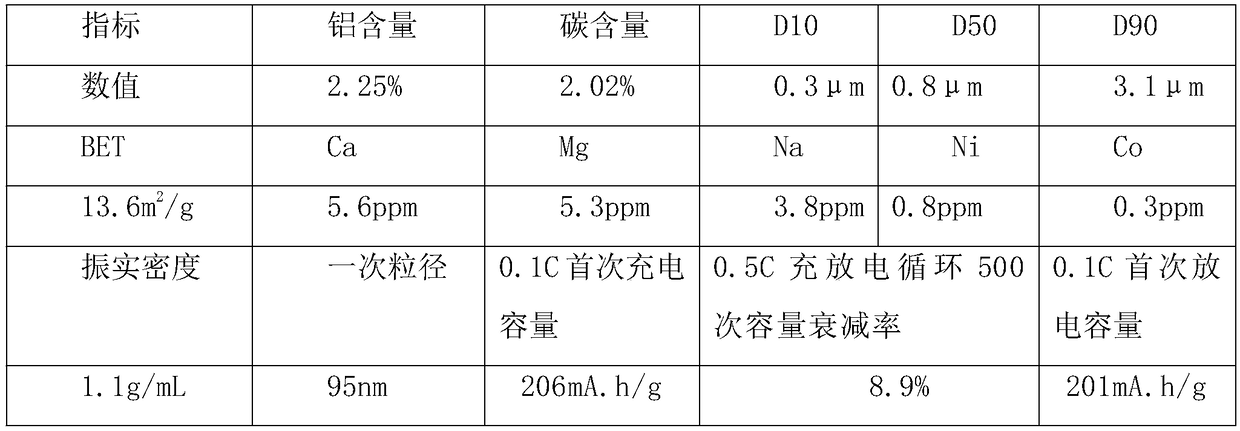
Embodiment 1
[0030] A kind of preparation method of lithium iron borate, it is the following steps:
[0031] (1) Mix the lithium borohydride solution and the ferric nitrate solution into a sealed high-pressure reactor, stir and react for 50 minutes at a temperature of 235°C and a pressure of 3 atmospheres, then release the pressure, and pour the derived gas with ferrous hydroxide Slurry absorption, then add aluminum citrate, stir and mix evenly, and then spray dry to obtain dry material;
[0032] (2) Sinter the dry material obtained in step (1) under an inert atmosphere. The calcination is divided into a heating section, a heat preservation section and a cooling section. The maintenance temperature in the section is 705°C, the residence time in the holding section is 17 hours, the cooling rate in the cooling section is 110°C / h, and the calcined material is obtained by cooling to a temperature below 60°C;
[0033] (3) The obtained calcined material is subjected to airflow crushing through ...
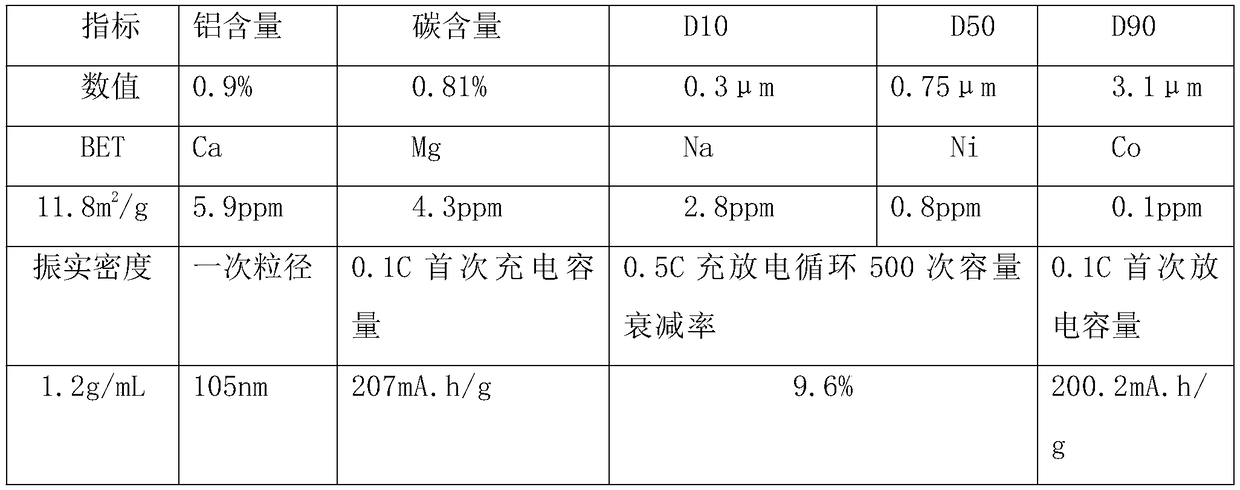
Embodiment 2
[0042] A kind of preparation method of lithium iron borate, it is the following steps:
[0043] (1) Mix the lithium borohydride solution and the ferric nitrate solution into a sealed high-pressure reactor, stir and react for 50 minutes at a temperature of 240°C and a pressure of 3.5 atmospheres, and then release the pressure. Slurry absorption, then add aluminum citrate, stir and mix evenly, and then spray dry to obtain dry material;
[0044] (2) The dry material obtained in step (1) is sintered under an inert atmosphere. The calcination is divided into a heating section, a heat preservation section and a cooling section. The maintenance temperature in the section is 706°C, the residence time in the holding section is 18 hours, the cooling rate in the cooling section is 107°C / h, and the calcined material is obtained by cooling to a temperature below 60°C;
[0045] (3) The obtained calcined material is subjected to airflow crushing through nitrogen gas, then sieved to remove iro...
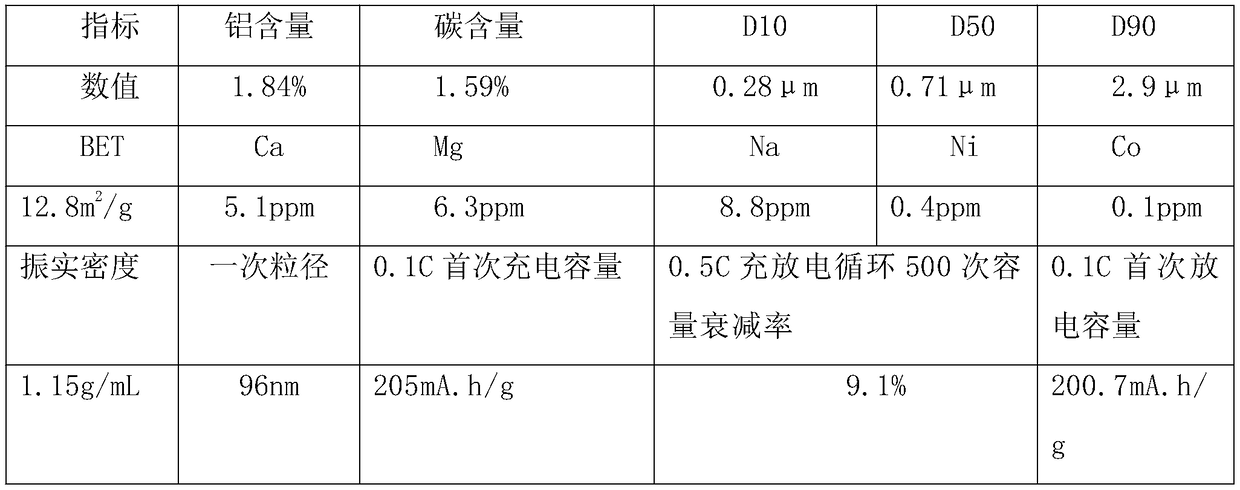
Embodiment 3
[0054] A kind of preparation method of lithium iron borate, it is the following steps:
[0055] (1) Mix lithium borohydride solution and ferric nitrate solution into a sealed high-pressure reaction kettle, stir and react for 45 minutes at a temperature of 240°C and a pressure of 3.5 atmospheres, and then release the pressure. Slurry absorption, then add aluminum citrate, stir and mix evenly, and then spray dry to obtain dry material;
[0056] (2) Sinter the dry material obtained in step (1) under an inert atmosphere. The calcination is divided into a heating section, a heat preservation section and a cooling section. The maintenance temperature in the section is 703°C, the residence time in the holding section is 19 hours, the cooling rate in the cooling section is 107°C / h, and the calcined material is obtained by cooling to a temperature below 60°C;
[0057] (3) The obtained calcined material is subjected to airflow crushing through nitrogen gas, then sieved to remove iron, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com