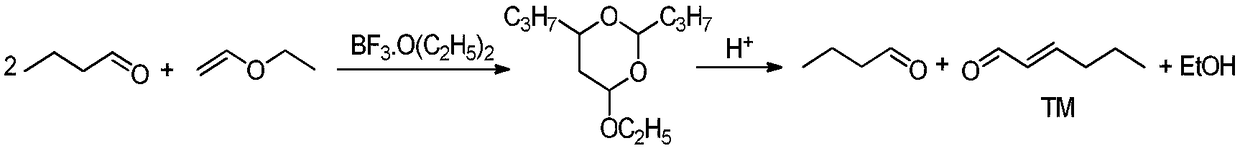
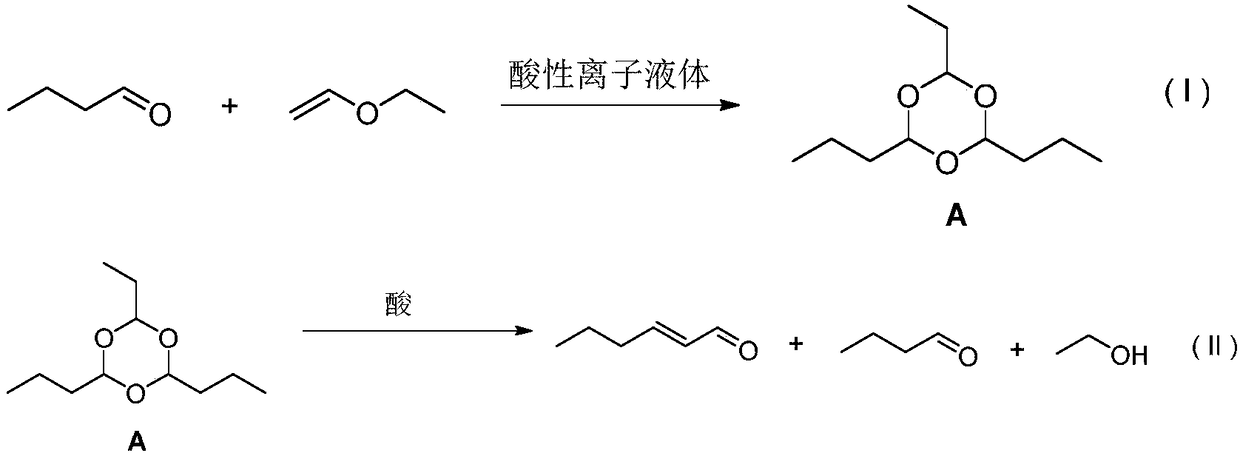
Method for synthesizing trans-2-hexenal
A technology of hexenal and reaction formula, applied in the field of synthesizing trans-2-hexenal, can solve the problems of unavailable raw materials, low reaction yield, high price, etc., achieve a recyclable environment, easy separation and recovery, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 15g ionic liquid [(CH 2 ) 3 SO 3 HMIM][HSO 4 ], 500g of vinyl ethyl ether and 1100g of n-butyraldehyde mixed solution was added dropwise at 25°C, stirred and reacted at 45°C for 1 hour after the dropwise addition, vacuum distillation after the reaction was completed, the water pump pressure was -0.1Mpa, the kettle temperature was 130°C, and the temperature At 125°C, 1582g of fractions were collected, the content of intermediate A was 84.3%, and the yield was 95.1%. The recovered ionic liquid catalyst weighed 16g in the still material.
[0027] Add 3164g of 0.5% dilute sulfuric acid solution and 1582g of the above-mentioned intermediate A with a content of 84.3% to the reaction bottle, and set up a reflux oil-water stratification device. Heating and normal pressure distillation, oil and water in the water separator are separated, the oil layer is recovered on the upper side and enters the oil layer receiving bottle, and the water layer is returned to the reaction ...
Embodiment 2
[0030] Add 10g ionic liquid [(CH 2 ) 3 SO 3 HMIM][CF 3 SO 3 ], 500g of vinyl ether and 1500g of n-butyraldehyde mixed solution was added dropwise at 0°C, after the dropwise addition was completed, the temperature was raised to 20°C and stirred for 6 hours, and after the reaction was completed, distillation under reduced pressure was carried out. The pressure of the water pump was -0.1Mpa, and the temperature of the kettle was 130°C. The temperature was 125° C., 1986 g of fractions were collected, the content of intermediate A was 69.3%, and the yield was 98.1%. The recovered ionic liquid catalyst weighed 11 g in the still material.
[0031] Add 397g of 25% dilute sulfuric acid solution and 1986g of the above-mentioned intermediate A with a content of 69.3% to the reaction bottle, and set up a reflux oil-water stratification device. Heating and normal pressure distillation, oil and water in the water separator are separated, the oil layer is recovered on the upper side and ...
Embodiment 3
[0034] Add 0.5 g ionic liquid [(CH 2 ) 3 SO 3 HMIM][CF 3 SO 3 ], 500g of vinyl ethyl ether and 2500g of n-butyraldehyde mixed solution was added dropwise at 15°C, after the dropwise addition was completed, the temperature was raised to 35°C and the reaction was stirred for 3 hours, and after the reaction was completed, distillation under reduced pressure was carried out. The pressure of the water pump was -0.1Mpa, and the temperature of the kettle was 130°C. The temperature was 125°C, 2995g of fractions were collected, the content of intermediate A was 20.9%, and the yield was 92.0%. The recovered ionic liquid catalyst weighed 0.6g in the still material.
[0035] Add 1000g of 5% dilute sulfuric acid solution and 2995g of the above-mentioned intermediate A with a content of 20.9% to the reaction flask, and set up a reflux oil-water stratification device. Heating and atmospheric pressure distillation, oil and water in the water separator are separated, the oil layer is recov...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com