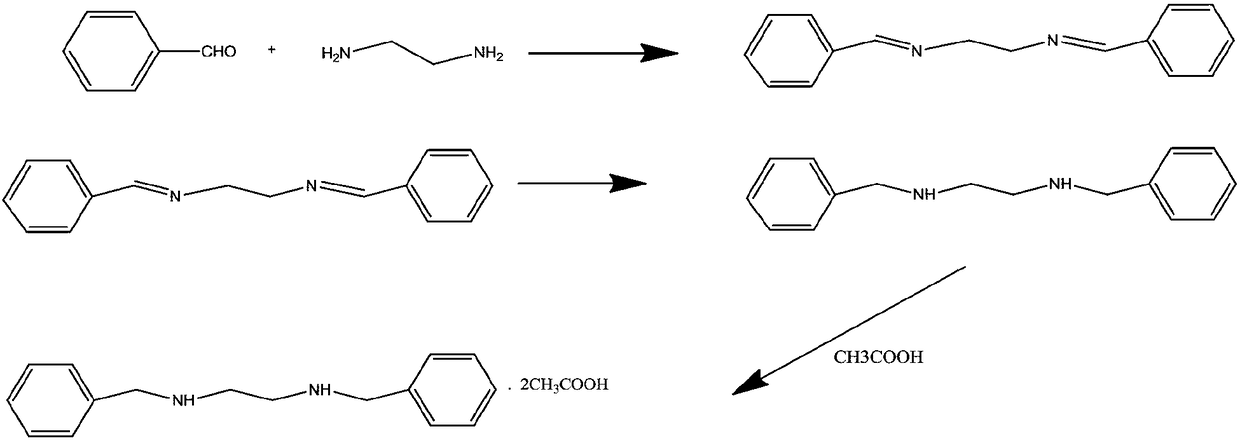
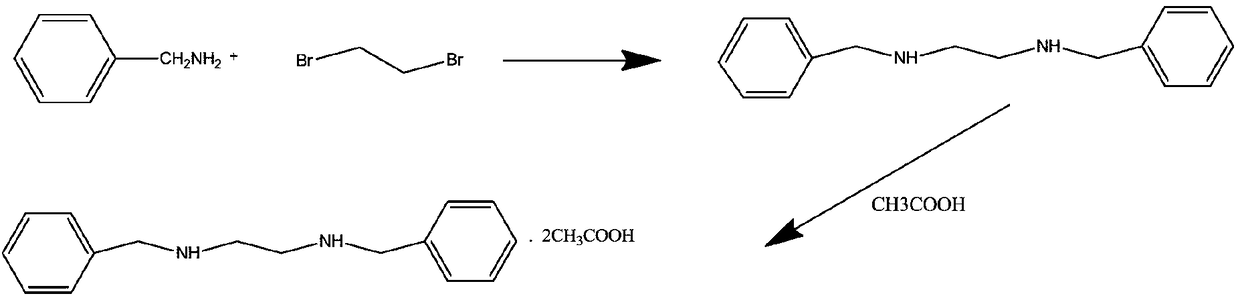
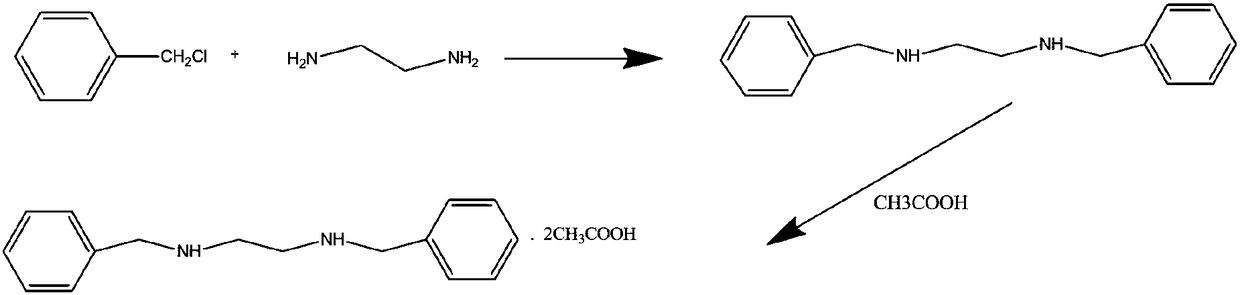
Green preparing method for high-purity N,N'-dibenzyl ethylenediamine diacetate
A technology of dibenzylethylenediamine hydrochloride and dibenzylethylenediamine is applied in the field of green preparation of high-purity N,N'-dibenzylethylenediaminediacetic acid, and can solve the harm to operators and the environment , by-products are difficult to handle, difficult to produce and store, etc., to achieve the effect of low production cost, reduction of by-products or waste, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1. Preparation of free base N,N'-dibenzylethylenediamine:
[0028] Add 420ml of drinking water to a 1000ml reaction bottle, put in 60g of N,N'-dibenzylethylenediamine hydrochloride, control the temperature of the material not to exceed 40°C; then control the temperature at 25°C and add 90g of ammonia water dropwise, and the dropwise addition is completed , continue to stir for 30 minutes; add 540g of dichloromethane, stir for 30 minutes, and let stand for 15 minutes. After the materials are separated, the lower organic layer is transferred to a 1000ml concentration bottle, and the temperature is controlled at 60°C. , to N, N'-dibenzylethylenediamine solution.
[0029] 2. Preparation of N,N'-dibenzylethylenediaminediacetic acid:
[0030] Put 420g of methyl acetate into the concentrated bottle, control the temperature at 30°C and dissolve it. After the dissolution time is 30 minutes, add 28g of acetic acid dropwise at 30°C to form a salt reaction. The dropwise addition t...
Embodiment 2
[0032] 1. Preparation of free base N,N'-dibenzylethylenediamine:
[0033] Add 420L of drinking water to the 1000L reaction kettle, put in 60kg of N,N'-dibenzylethylenediamine sulfate, control the temperature of the material not to exceed 40°C; then control the temperature at 40°C and add 90kg of sodium hydroxide dropwise, and the dropwise addition is completed , continue to stir for 60 minutes; add 540 kg of chloroform, stir for 60 minutes, and let stand for 20 minutes. After the materials are separated, the lower organic layer is transferred to a 1000L concentration kettle, and the temperature is controlled at 40 ° C. Steam 480 kg of organic phase chloroform , to N, N'-dibenzylethylenediamine solution.
[0034] 2. Preparation of N,N'-dibenzylethylenediaminediacetic acid:
[0035] Put 420kg of methyl acetate into the concentrated reaction tank, control the temperature at 60°C to dissolve, and after the dissolution time is 90 minutes, add 28kg of acetic acid dropwise at 0°C to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com