Space-conjugated organic molecule based on hexaarylbenzene skeleton and preparation and application thereof
A technology of hexaarylbenzene and organic molecules, which is applied in the field of organic molecules with steric conjugation effects and its preparation, can solve the problems of blocked charge transmission channels, and the conductivity of rod-shaped molecules has not reached industrial application, achieving simple structure, Excellent effect, the effect of improving electrical conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
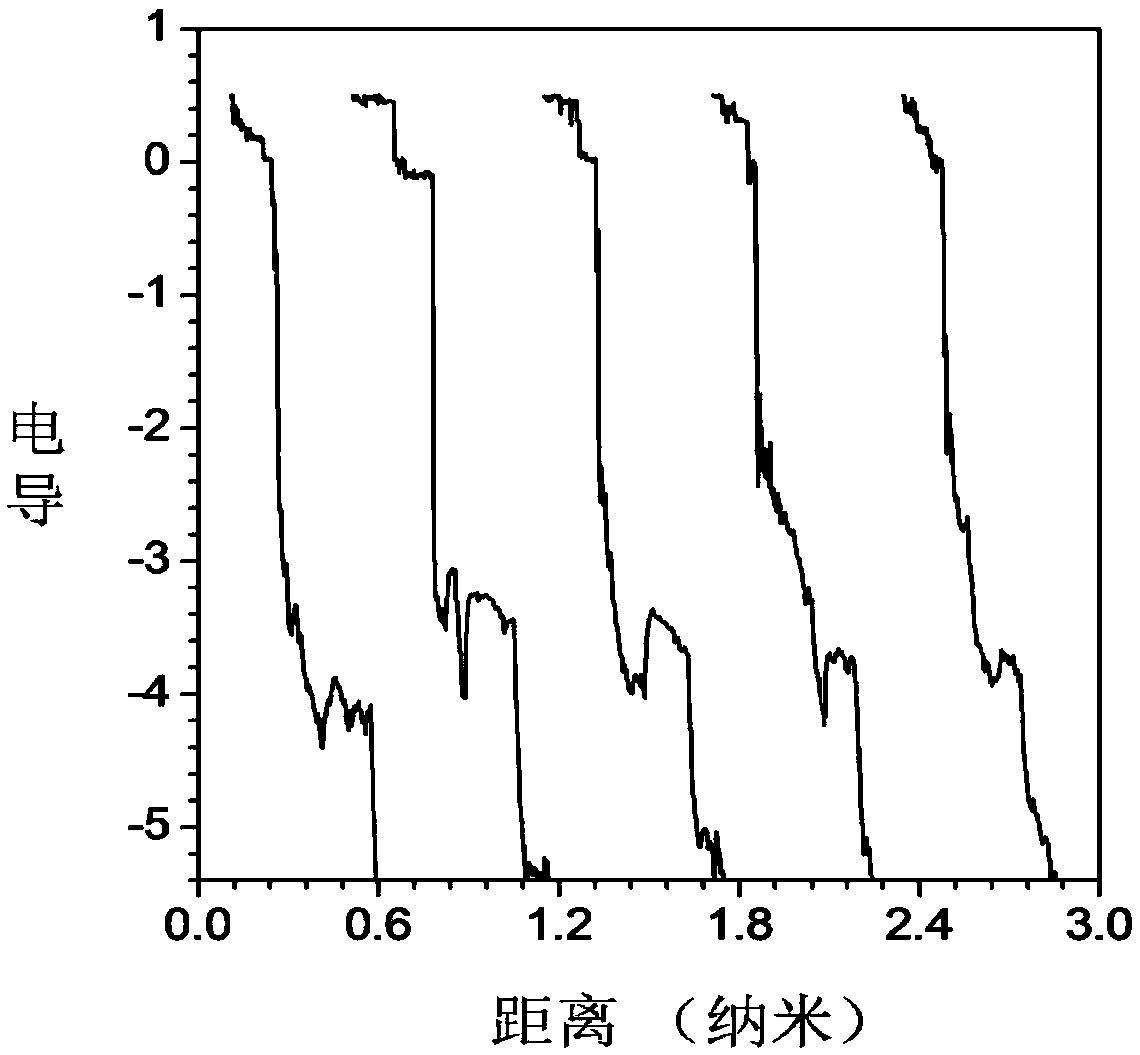
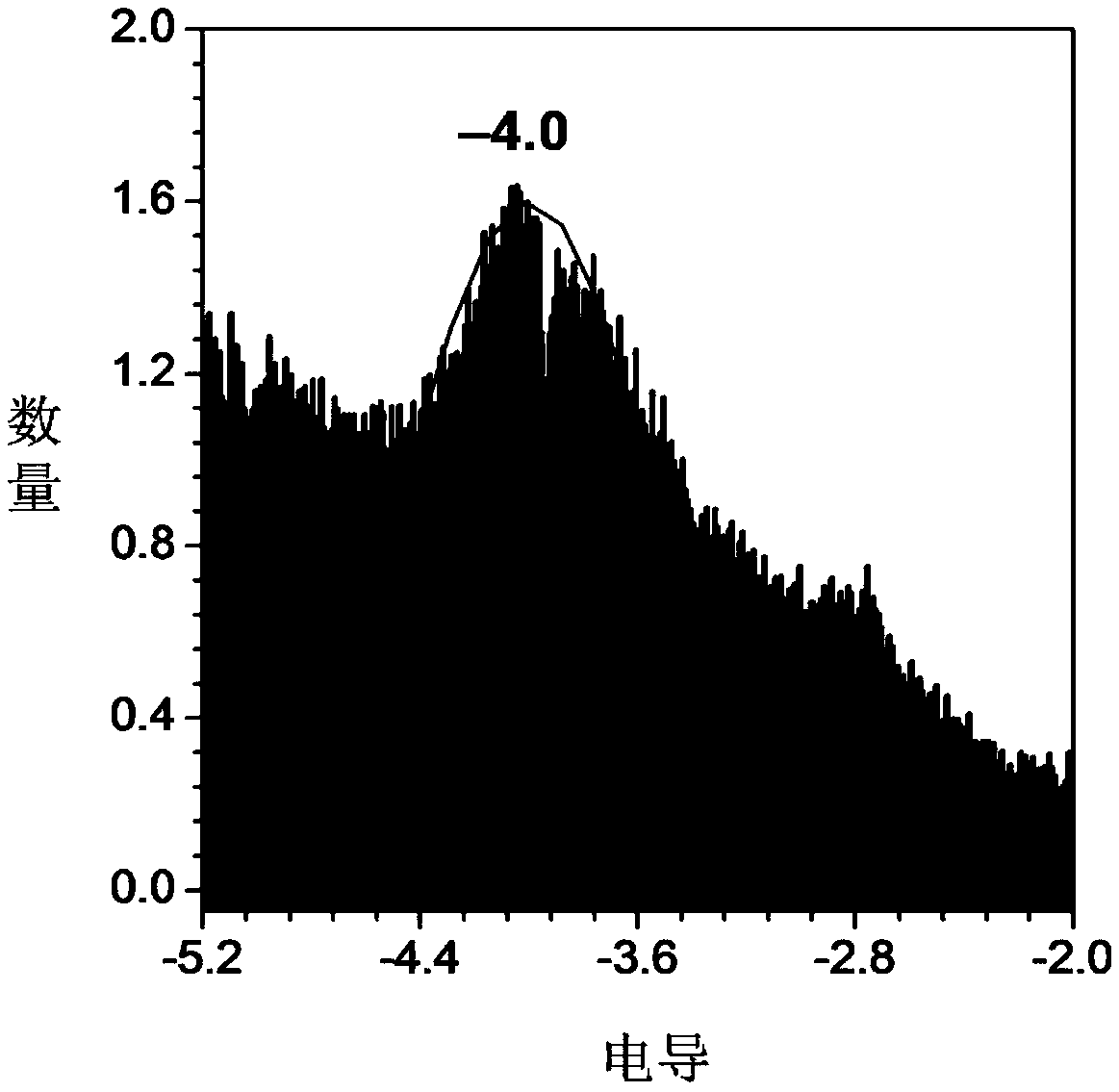
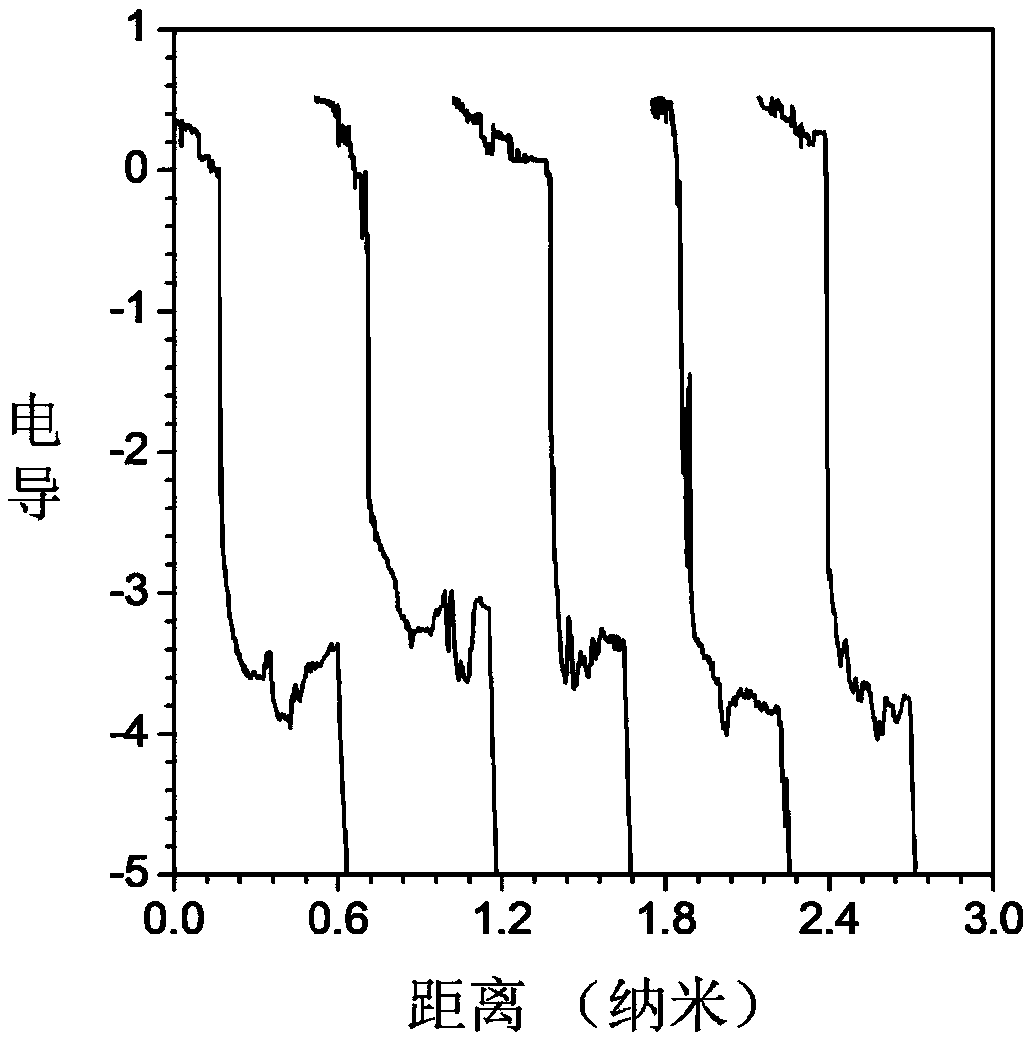
Image
Examples
Embodiment 1
[0040] Embodiment 1: Preparation of spatially conjugated organic molecule [HP(OMe)B-SMe] based on hexaarylbenzene:
[0041]
[0042] Reaction equation (1):
[0043]
[0044]
[0045] (1) Add dicyclohexylcarbodiimide (DCC) (6.19g, 30mmol) and 4-dimethylaminopyridine (916mg, 7.5mmol) to the reaction vessel, pump and exchange gas three times, add dichloromethane under nitrogen atmosphere Methane 60mL, dropwise add a dichloromethane solution (dichloromethane 60mL) of 4-bromophenylacetic acid (compound 1, 30mmol), continue to react for 24 hours after the dropwise addition, concentrate to remove the solvent after the reaction is completed, and separate by column chromatography The intermediate product 2 (compound 2) was obtained by purification with a yield of 60%;
[0046] (2) Add intermediate product 2 (3.69g, 10mmol), 4,4'-dimethoxyphenol ester (compound 3) (2.7g, 10mmol) and potassium hydroxide (0.168g, 3mmol) into the reaction flask , pumped and changed three times, ...
Embodiment 2
[0050] Example 2: Spatically conjugated organic molecules based on hexaarylbenzene [HP(OMe) 3 Preparation of B-SMe]
[0051]
[0052] Reaction equation (2):
[0053]
[0054] (1) Add raw material 1 (1-bromo-3,4,5-trimethoxybenzene) (2.47g, 10mmol), raw material 2 (butynedic acid) (0.57g, 5mmol), bis(triphenylphosphine ) palladium dichloride (0.35g, 0.5mmol), 1,4-bis(diphenylphosphine)butane (0.21g, 0.5mmol), 1,8-diazabicyclo[5.4.0]undecane -7-ene (1.52g, 10mmol) was added to the reaction flask, pumped and ventilated three times, added 60mL dimethyl sulfoxide solvent, and reacted at 115°C for 12 hours. After the reaction was completed, poured into 200mL distilled water and dichloromethane Three times, washed three times with water, dried, and the solvent was removed by rotary evaporation, separated and purified by column chromatography to obtain a light yellow intermediate product 3 (compound 3), with a yield of 55%;
[0055] (2) Add intermediate product 3 (1.79g, 5mmo...
Embodiment 3
[0061] Example 3: Sterically conjugated organic molecules based on hexaarylbenzene [HP 2 T 2 Preparation of B-SMe]
[0062]
[0063] Reaction equation (3):
[0064]
[0065](1) Add dicyclohexylcarbodiimide (DCC) (6.19g, 30mmol) and 4-dimethylaminopyridine (916mg, 7.5mmol) to the reaction vessel, pump and exchange gas three times, add dichloromethane under nitrogen atmosphere Methane 60mL, dropwise add a dichloromethane solution (dichloromethane 60mL) of 4-bromophenylacetic acid (compound 1, 30mmol), continue to react for 24 hours after the dropwise addition, concentrate to remove the solvent after the reaction is completed, and separate by column chromatography Purified to obtain intermediate product 2 with a yield of 60%;
[0066] (2) Add intermediate product 2 (3.69g, 10mmol), thienoin (compound 3) (2.2g, 10mmol) and potassium hydroxide (0.168g, 3mmol) into the reaction flask, pump and change gas three times, under nitrogen protection Inject solvent ethanol (30mL),...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductance | aaaaa | aaaaa |
| electrical conductance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com