Cobalt-nitrogen co-doped porous carbon material and preparation and application thereof
A porous carbon material, co-doping technology, applied in electrical components, battery electrodes, circuits, etc., can solve the problems of poor conductivity, limitation, low volume activity, etc., to improve carbon yield and nitrogen fixation rate, aerobic reduction catalysis Activity and cycle stability, effect of simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
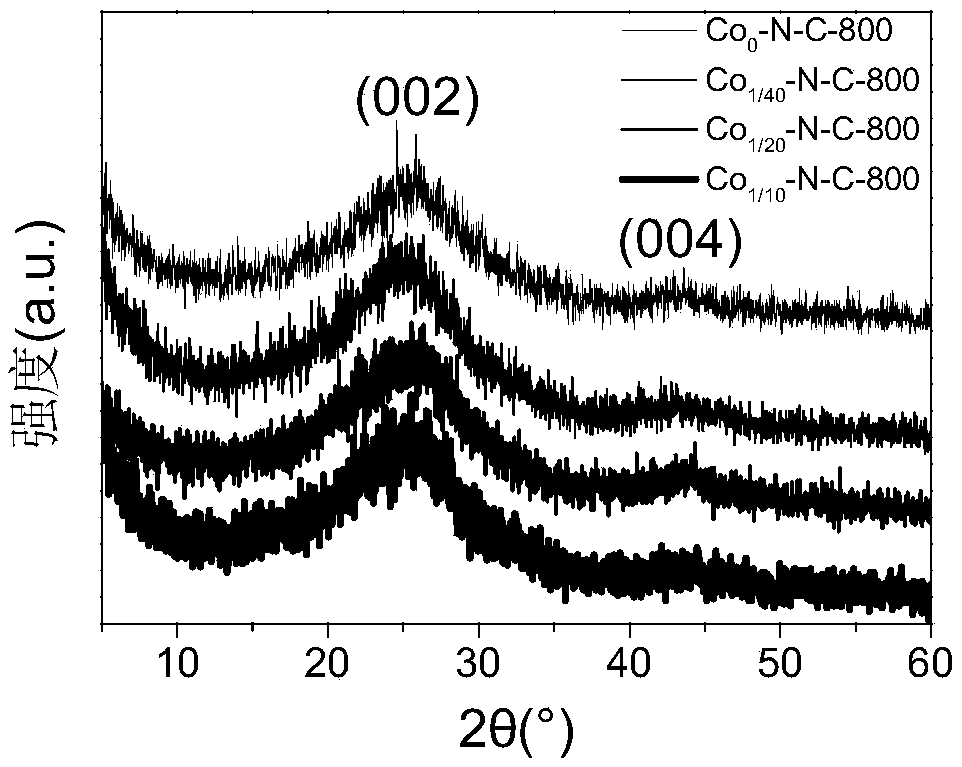
Embodiment 1
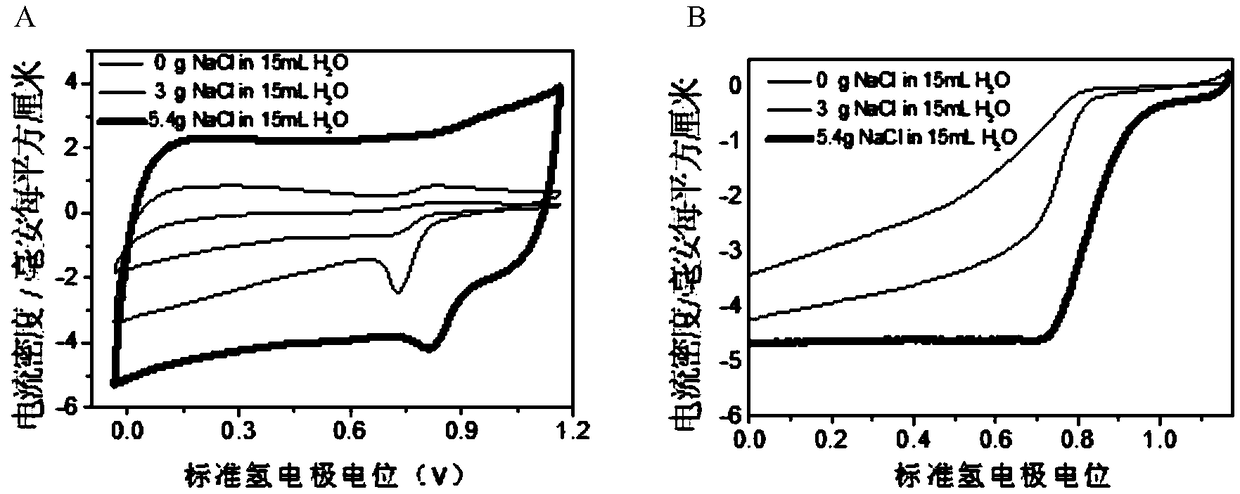
[0038] (1) Prepare 15ml of sodium chloride solution with saturated concentration (5.4g / 15mL) in deionized water.
[0039] (2) Disperse 1 g of riboflavin in the above solution, and ultrasonically disperse to make it evenly mixed.
[0040] (3) Add 0.05 g of cobalt acetate in addition to allow it to fully dissolve.
[0041] (4) Pour 30mL of liquid nitrogen into the solution of (3) and let stand for 10min.
[0042] (5) Transfer the system (4) into a freeze dryer for drying and then dry it in a vacuum oven at 80° C. for 48 hours to obtain the corresponding carbonized precursor.
[0043] (6) The sample was carbonized at 800°C for 2 hours under an argon atmosphere, then washed with dilute hydrochloric acid and deionized water, and finally the solid powder was left by suction filtration and dried in a vacuum oven to obtain the corresponding cobalt-nitrogen co-doped catalyst.
Embodiment 2
[0045] (1) Prepare 15ml of sodium chloride solution in deionized water with a concentration of 3g / 15ml.
[0046] (2) Disperse 1 g of riboflavin in the above solution, and ultrasonically disperse to make it evenly mixed.
[0047] (3) Add 0.05 g of cobalt acetate in addition to allow it to fully dissolve.
[0048] (4) Pour 30mL of liquid nitrogen into the solution of (3) and let stand for 10min.
[0049] (5) Transfer the system (4) into a freeze dryer for drying and then dry it in a vacuum oven at 80° C. for 48 hours to obtain the corresponding carbonized precursor.
[0050] (6) The sample was carbonized at 800°C for 2 hours under an argon atmosphere, then washed with dilute hydrochloric acid and deionized water, and finally the solid powder was left by suction filtration and dried in a vacuum oven to obtain the corresponding cobalt-nitrogen co-doped catalyst.
Embodiment 3
[0051] Embodiment 3 (comparative example)
[0052](1) Disperse 1 g of riboflavin in 15 ml of water, and ultrasonically disperse to mix evenly.
[0053] (2) Add 0.05 g of cobalt acetate in addition to allow it to fully dissolve.
[0054] (3) Pour 30mL of liquid nitrogen into the solution of (3) and let stand for 10min.
[0055] (5) Transfer the system (4) into a freeze dryer for drying and then dry it in a vacuum oven at 80° C. for 48 hours to obtain the corresponding carbonized precursor.
[0056] (6) The sample was carbonized at 800°C for 2 hours under an argon atmosphere, then washed with dilute hydrochloric acid and deionized water, and finally the solid powder was left by suction filtration and dried in a vacuum oven to obtain the corresponding cobalt-nitrogen co-doped catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com