Method for producing methyl acetate from synthesis gas by one-step method
A technology of methyl acetate and synthesis gas, which is applied in the direction of carbon monoxide or formate reaction preparation, organic chemistry, etc., can solve the problem of decreased activity of dimethyl ether carbonylation reaction, achieve simple and controllable preparation process, and shorten reaction steps , The effect of low catalyst cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
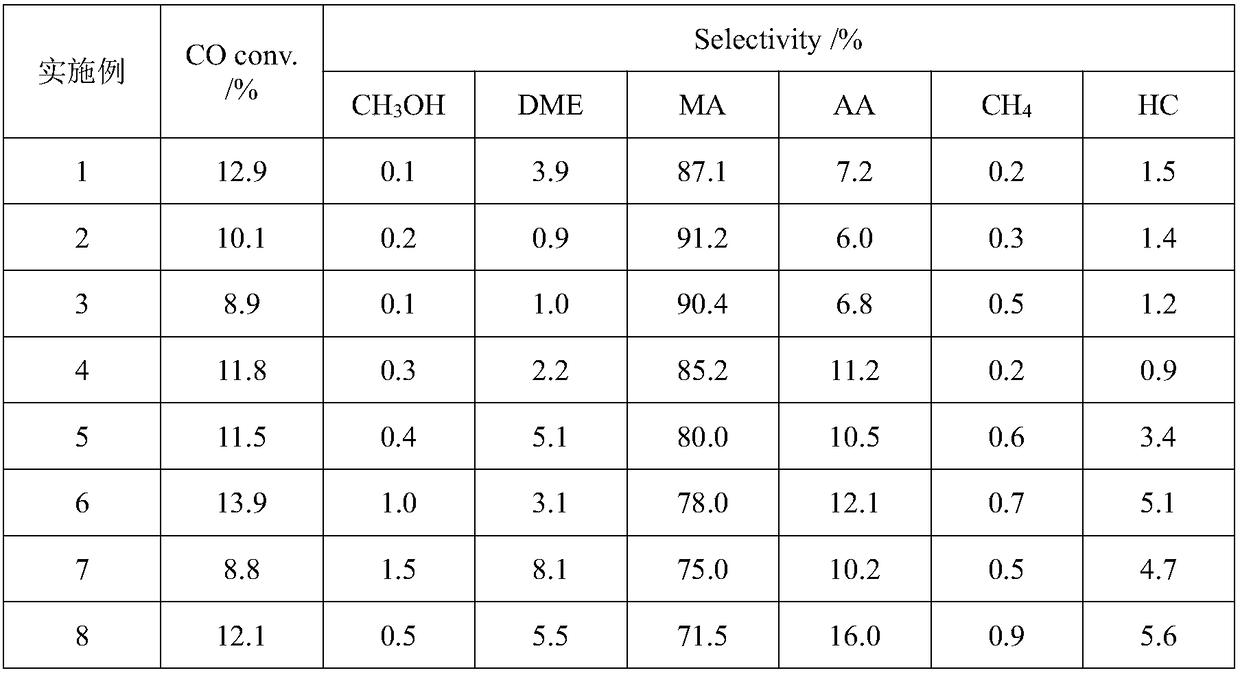
[0027] Weigh 0.2g Cu-ZnO (Cu mass fraction is 40%) and 0.4g HZSM-5 (Si / Al=25), shape them separately, carry out physical mixing, then weigh 0.6g HMOR (Si / Al=13) . The physically mixed catalyst is placed in the upper layer of the fixed bed reactor, and the HMOR is placed in the lower layer. Inject 5%H 2 / Ar mixed gas, heated to 300°C at a heating rate of 10°C / min to pretreat the catalyst, and kept for 300min. After being lowered to room temperature, synthesis gas was introduced, where H 2 The / CO ratio is 2 and the airspeed is 1000h -1 1. Under the condition that the reaction temperature is 220° C. and the reaction pressure is 3.0 MPa, the product is obtained through catalyst bed reaction. The reaction products and feed gas were analyzed online by gas chromatography. The specific reaction performance results are listed in Table 1.
Embodiment 2
[0029] Weigh 0.2g Cu-ZnO (Cu mass fraction is 40%) and 0.4g HBeta (Si / Al=50), shape them separately, carry out physical mixing, and weigh 0.6g HMOR (Si / Al=13). The physically mixed catalyst is placed in the upper layer of the fixed bed reactor, and the HMOR is placed in the lower layer. Inject 5%H 2 / Ar mixed gas, heated to 300°C at a heating rate of 10°C / min to pretreat the catalyst, and kept for 300min. After being lowered to room temperature, synthesis gas was introduced, where H 2 / CO ratio is 1, at airspeed 1500h -1 1. Under the condition that the reaction temperature is 200° C. and the reaction pressure is 3.0 MPa, the product is obtained through catalyst bed reaction. The reaction products and feed gas were analyzed online by gas chromatography. The specific reaction performance results are listed in Table 1.
Embodiment 3
[0031] Weigh 0.2g ZnO-Cr respectively 2 o 3 (Zn mass fraction is 50%) and 0.4g HY (Si / Al=5.5), after molding respectively, carry out physical mixing, and weigh 0.6g HZSM-35 (Si / Al=12). The physically mixed catalyst is placed in the upper layer of the fixed bed reactor, and HZSM-35 is placed in the lower layer. Inject 5%H 2 / Ar mixed gas, heated to 300°C at a heating rate of 10°C / min to pretreat the catalyst, and kept for 300min. After being lowered to room temperature, synthesis gas was introduced, where H 2 / CO ratio is 1, at airspeed 1500h -1 1. Under the condition that the reaction temperature is 250° C. and the reaction pressure is 4.0 MPa, the product is obtained through catalyst bed layer reaction. The reaction products and feed gas were analyzed online by gas chromatography. The specific reaction performance results are listed in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com