Micromolecule organic solar energy battery materials based on a heterocycle containing boron and nitrogen and a preparation method thereof
A technology of solar cells and small molecules, applied in organic chemistry, chemical instruments and methods, circuits, etc., can solve problems such as differences in properties of organic molecules, achieve good planar performance, improve conversion efficiency, and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Synthesis of Acceptor Materials Containing Boron Heterocycle BN-Ph
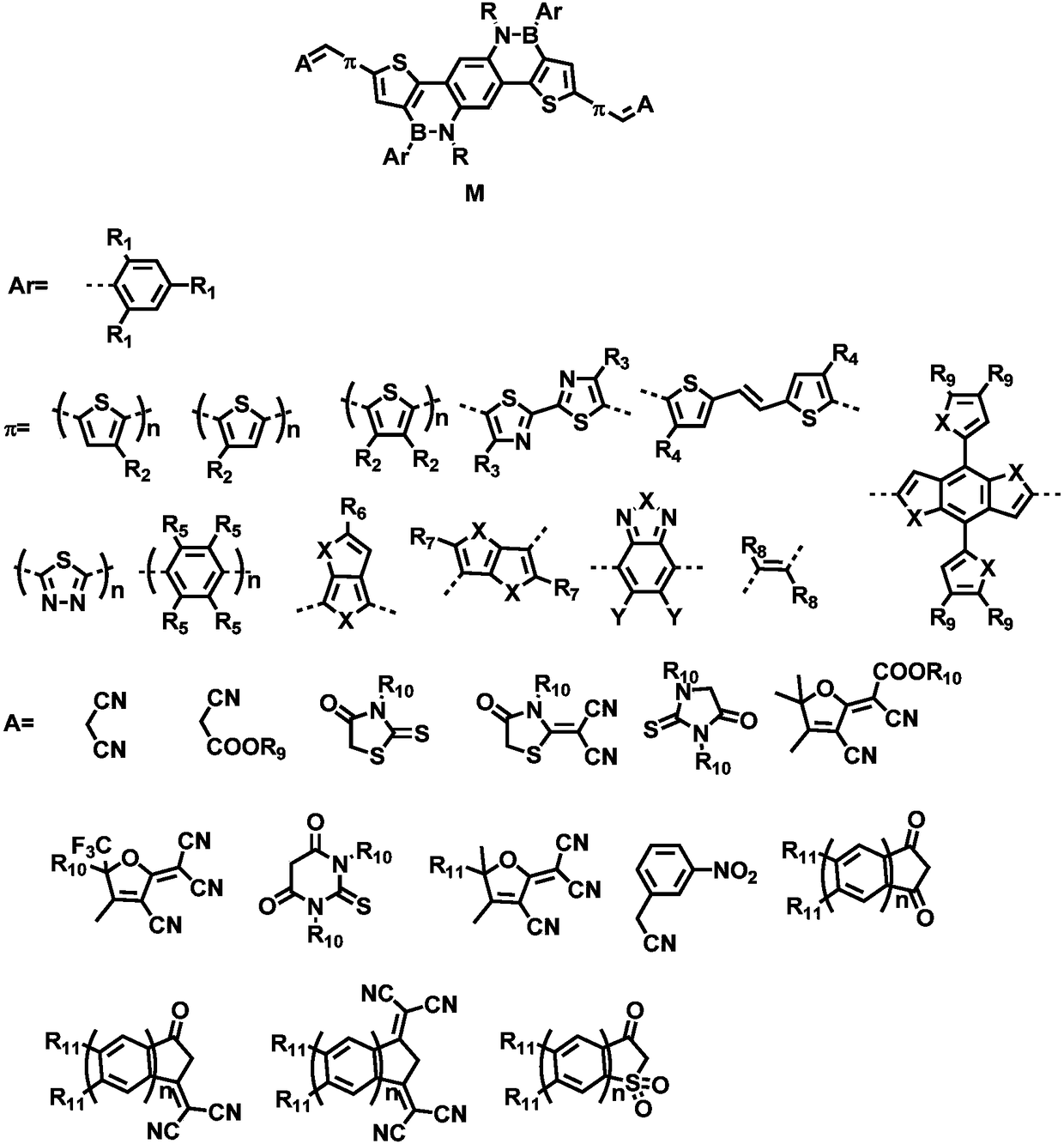
[0034] The synthesis steps are shown in the figure below. The previous synthesis work of this kind of small molecule acceptor material is the same. The borane substitution introduces different substituted phenyl groups, the bridging unit introduces different π units, and the Knoevenagel condensation reaction introduces different Electron-withdrawing groups, thereby producing small molecule acceptor materials with different band gaps and energy levels. Therefore, in the specific implementation case, Ar is the benzene ring, and the electron-withdrawing group A is 3-(dicyanomethylene) indigo-1 - Example of the synthesis of small molecule acceptor materials for ketones.
[0035]
[0036] The first step, by 2,5-dibromo-p-phenylenediamine as the raw material synthetic dibromoaniline substituted, as shown in the figure, the specific steps are: 2,5-dibromo-p-phenylenediamine (5g) is added to the In water t...
Embodiment 2
[0048] Taking the material obtained in Example 1 as an example to illustrate the application of such boron-nitrogen materials as small molecule acceptors in organic solar cell devices
[0049] The following example will illustrate the boron-nitrogen-containing heterocyclic small molecule organic solar material proposed in the present invention and its application process in organic photoelectric devices, but the present invention is not limited to the examples given.
[0050] The specific preparation process of the device is as follows:
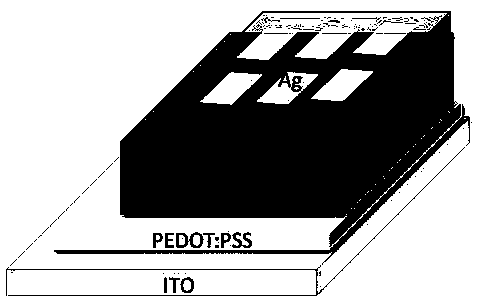
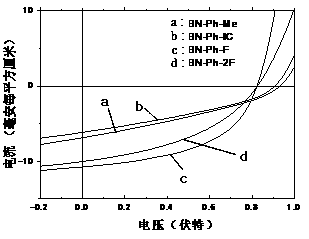
[0051] Spin-coat a 40nm PEDOT:PSS hole transport layer on ITO, then spin-coat a PTB7-Th and BN-Ph SM blend photoactive layer of about 100nm, and then spin-coat an amino-based polyfluorene of about 5nm The quaternary ammonium bromide salt (PFN-Br) was used as the cathode interface layer, and then a 100 nanometer Ag layer was evaporated to complete the preparation of the device. The J-V curve test is carried out, and the relevant parameters of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com