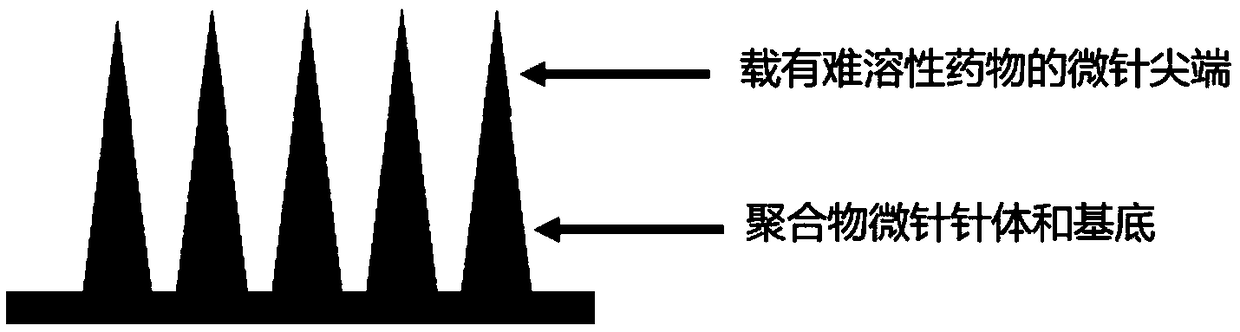
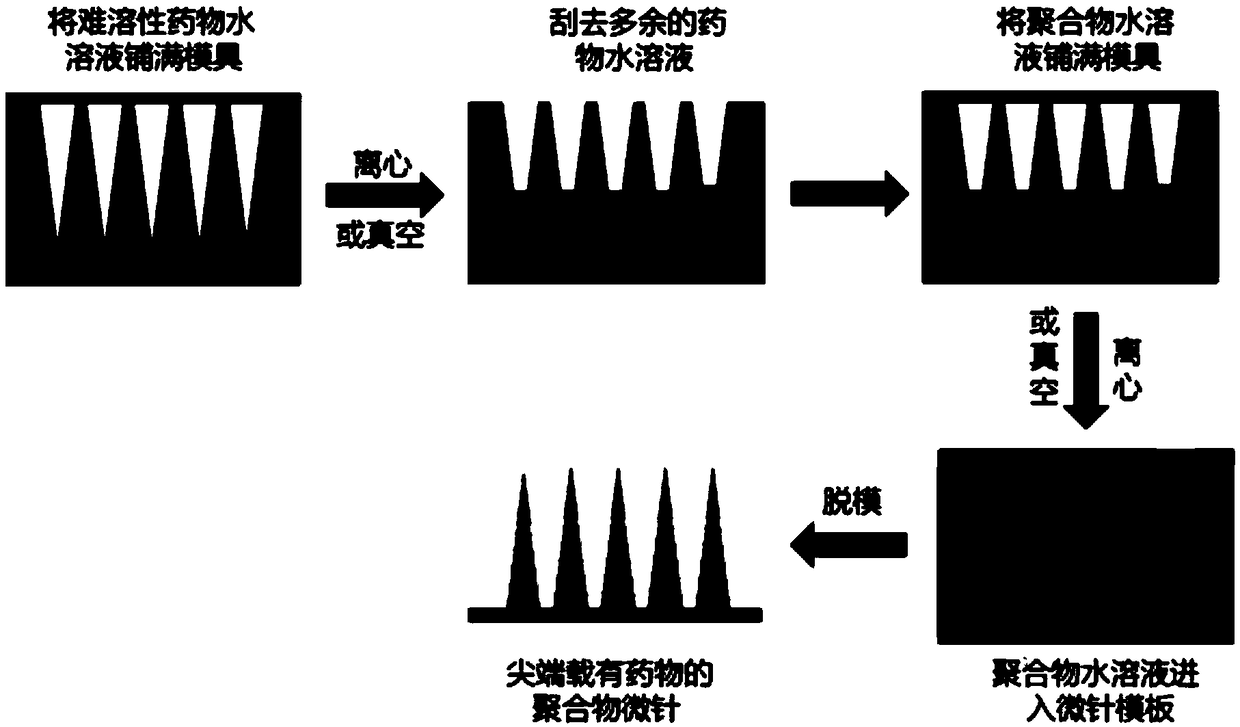
Soluble microneedle supported with hydrophobic drug and preparation method thereof
A hydrophobic drug, soluble technology, applied in the direction of drug devices, microneedles, drug delivery, etc., can solve the problem of not guaranteeing complete removal of organic solvents, easy residual organic solvents in microneedles, etc., to achieve good biocompatibility and improve water solubility. The effect of improving the solubility and improving the solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] This embodiment comprises the following steps:
[0030] 1. Stir and mix the PDMS precursor (Sylgard 184) and curing agent (mass ratio: 10:1), and inject it into a container with a microneedle male mold (the length of the microneedle is 50 μm) after the air bubbles are eliminated, and heat it to make it Completely cured, after curing, the microneedle positive mold and PDMS were separated to obtain the PDMS microneedle negative template.
[0031]2. Prepare an aqueous solution of triamcinolone acetonide (a hydrophobic hormone drug), wherein the content of triamcinolone acetonide in the drug solution is 0.1 wt%, and the content of dioleoyl lecithin is 0.01 wt%. Take hyaluronic acid powder, dissolve it in water, and magnetically stir for several hours to obtain a hyaluronic acid aqueous solution with a mass fraction of 60%.
[0032] 3. Add the triamcinolone acetonide aqueous solution into the PDMS mold, make the drug solution enter the tip of the mold under vacuum negative ...
Embodiment 2
[0035] This embodiment comprises the following steps:
[0036] 1. Stir and mix the PDMS precursor (Sylgard 184) and curing agent (mass ratio: 10:1). After the air bubbles are eliminated, inject it into a container with a microneedle male mold (the length of the microneedle is 900 μm), and heat it to make it Completely cured, after curing, the microneedle positive mold and PDMS were separated to obtain the PDMS microneedle negative template.
[0037] 2. Prepare an aqueous solution of methotrexate, wherein the content of methotrexate in the drug solution is 15 wt%, and the content of β-cyclodextrin is 20 wt%. Take chondroflavin powder, dissolve it in water, and magnetically stir for several hours to obtain a chondroflavin aqueous solution with a mass fraction of 30%.
[0038] 3. Add the methotrexate aqueous solution into the PDMS mold, make the drug solution enter the tip of the mold under vacuum negative pressure, and recover the excess drug solution on the surface of the temp...
Embodiment 3
[0041] This embodiment comprises the following steps:
[0042] 1. Stir and mix the PDMS precursor (Sylgard 184) and curing agent (mass ratio: 10:1). After the air bubbles are eliminated, pour it into a container with a microneedle male mold (the length of the microneedle is 2500 μm), and heat it to make it Completely cured, after curing, the microneedle positive mold and PDMS were separated to obtain the PDMS microneedle negative template.
[0043] 2. Prepare an aqueous solution of levofloxacin, wherein the content of levofloxacin in the drug solution is 30 wt%, and the content of polysorbate is 40 wt%. Take polyvinylpyrrolidone powder, dissolve it in water, and stir it magnetically for several hours to obtain an aqueous solution of polyvinylpyrrolidone with a mass fraction of 5%.
[0044] 3. Add the levofloxacin aqueous solution into the PDMS mold, make the drug solution enter the tip of the mold under centrifugal action, and recover the excess drug solution on the surface o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com