Method for jointly removing phosphine by aid of complexation systems and jet flow by means of oxidizing
A phosphine and system technology, applied in chemical instruments and methods, separation methods, gas treatment, etc., can solve the problems of potential safety hazards, blackening of waste acid, blackening of sulfuric acid, etc., to improve safety and corrosion Low, cost-saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
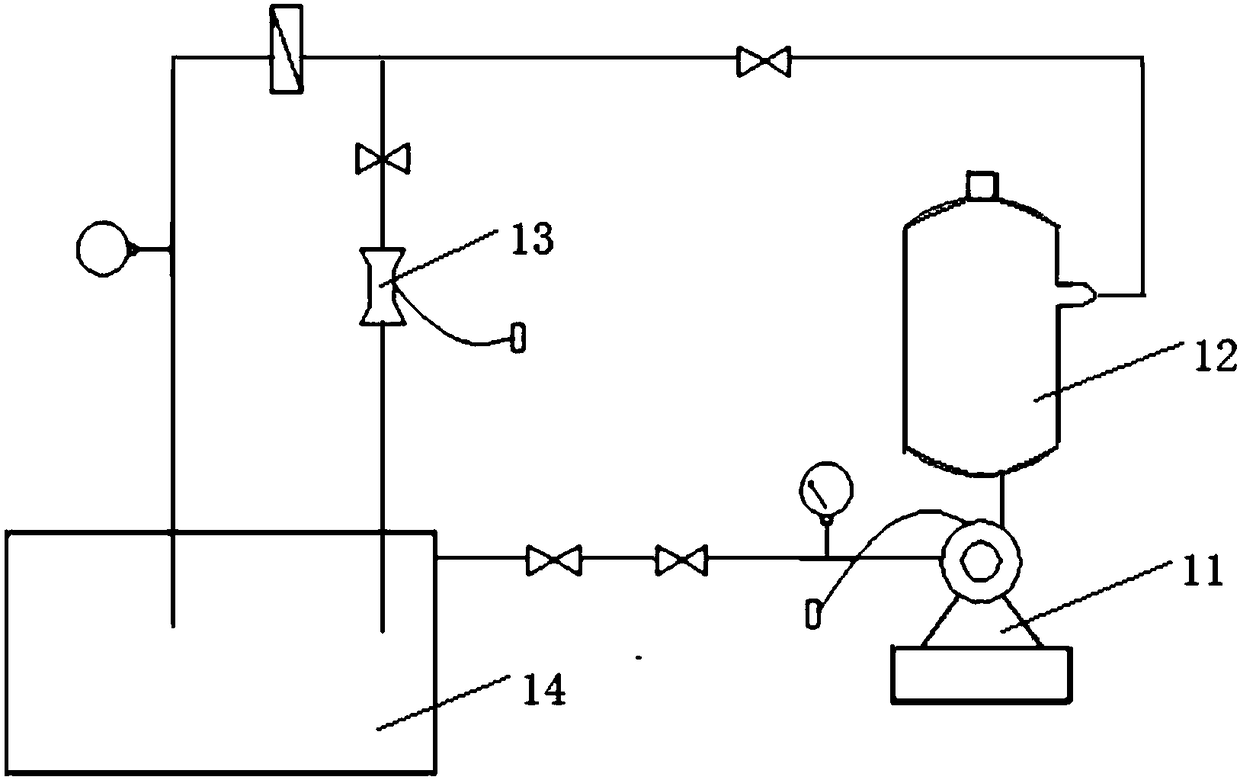
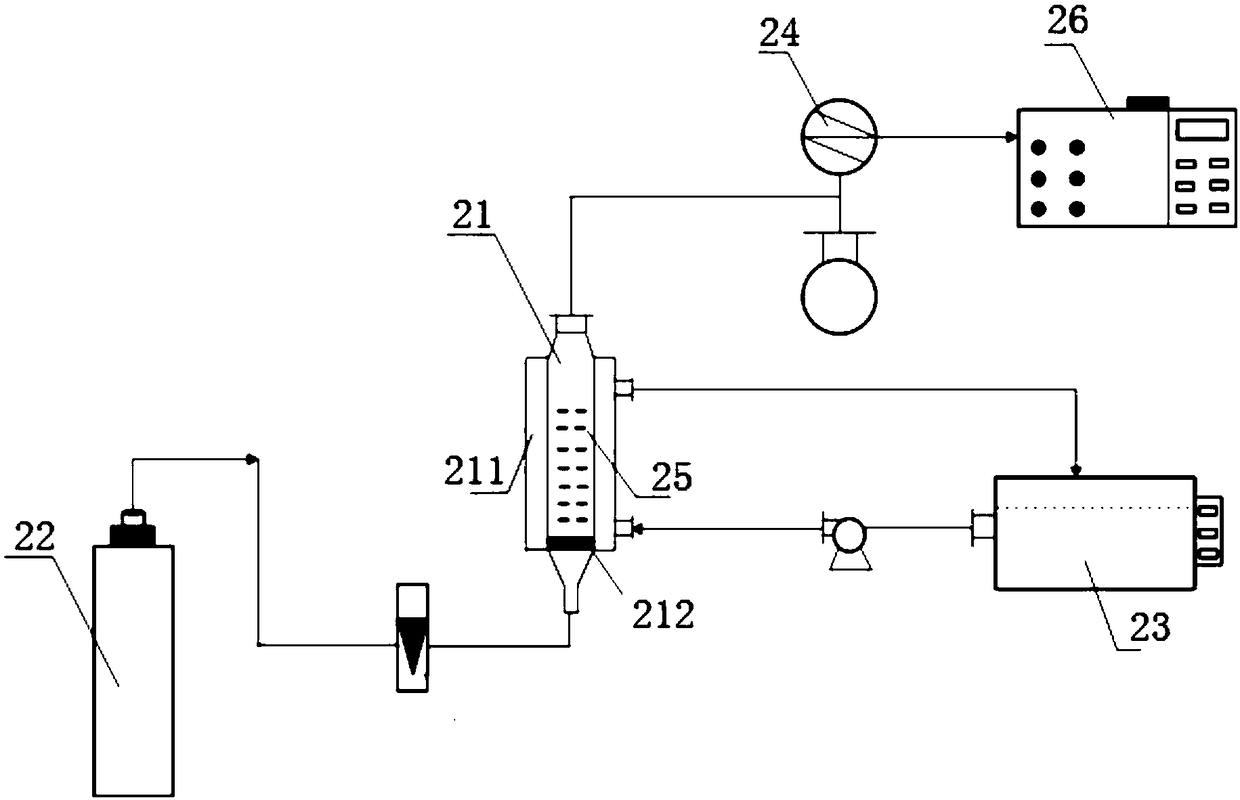
[0090] Weigh 1.76g Fe 2 (SO 4 ) 3 , 2.57g EDTA is added to 14.83mL 85% H 3 PO 4 After stirring to dissolve, dilute with pure water to 51mL(nEDTA:nFe 3+ =1:1); After mixing, pour it into a glass reaction device, and finally add 9mL of freshly produced nano-microbubble water, control the reaction temperature to maintain at 40°C, and after sealing, open the phosphine hydrogen bottle with a concentration of 1000ppm to start ventilation. And adjust the gas flow to stabilize at 20mL / min.
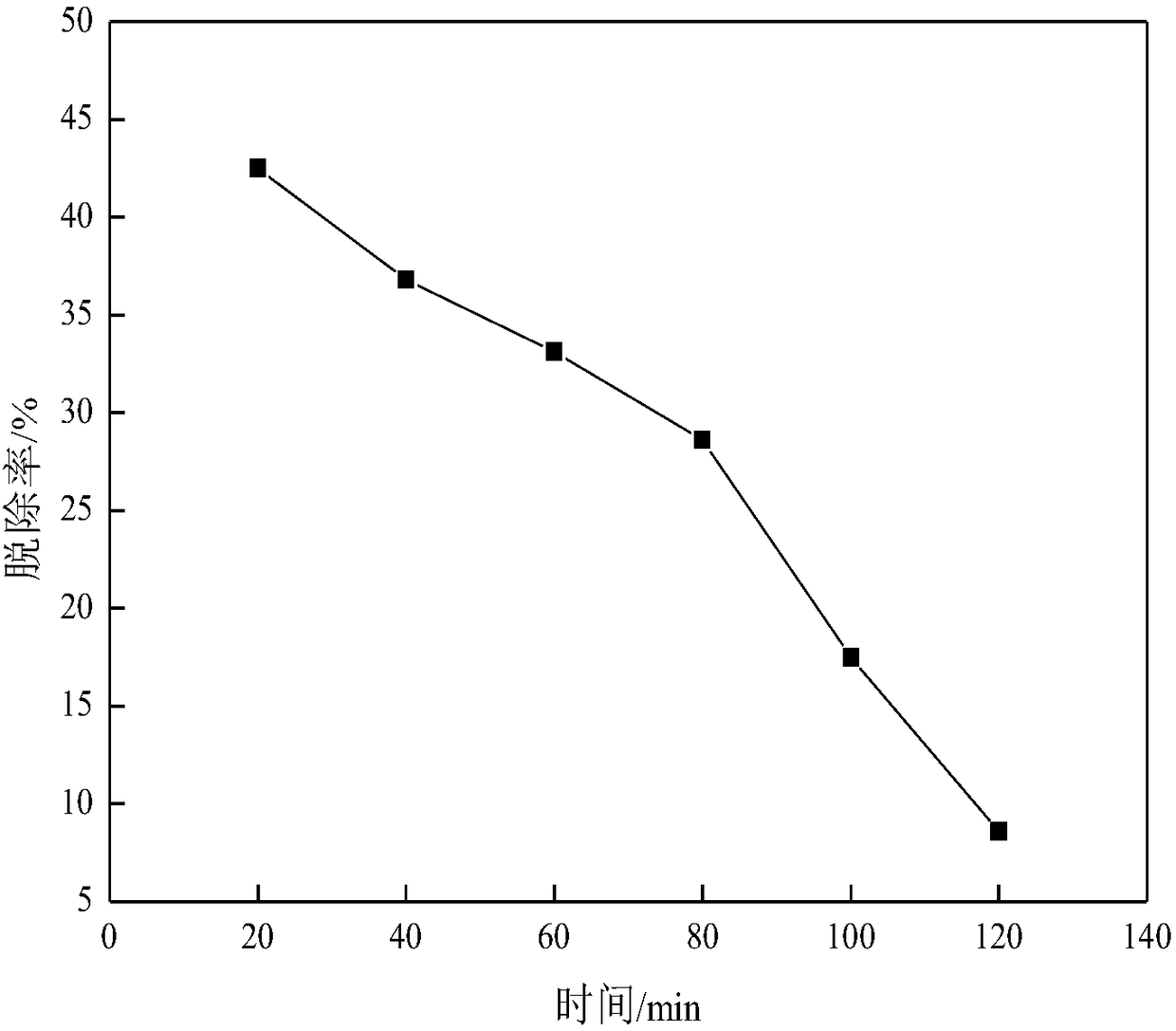
[0091] Such as image 3 As shown, the reaction liquid can react with phosphine, and the removal rate reaches the highest when the dephosphorization starts, which can reach about 45%. The reaction solution has an obvious effect on the removal of high-content phosphine, indicating that it can be applied to the removal of phosphine in the form of impurities in acetylene gas. Among them, the removal rate η is obtained by the following formula:
[0092]
[0093] C 0 Is the concentration of phosphine in ppm...
Embodiment 2
[0095] Weigh 1.76g Fe 2 (SO 4 ) 3 , 2.57g EDTA is added to 14.83mL 85% H 3 PO 4 After stirring to dissolve, dilute with pure water to 51mL(nEDTA:nFe 3+ =1:1), mix well and pour it into a glass reaction device, and finally add 9mL of freshly produced nanobubble water, control the reaction temperature at 40℃, 45℃ and 50℃ respectively, after sealing, open the phosphorus with a concentration of 1000ppm The hydrogen gas cylinder was ventilated, and the gas flow rate was adjusted to stabilize at 20 mL / min.
[0096] Such as Figure 4 As shown, as the temperature increases, the initial dephosphorization rate of the complexed iron and jet combined system also increases. The maximum dephosphorization rate of the system can reach 60% at 50℃, and the dephosphorization rate is faster than 40℃ and 45℃ over time; at 40℃, the dephosphorization rate can be maintained at a better level for a longer time (43% ~33%).
Embodiment 3
[0098] Weigh 1.76g Fe 2 (SO 4 ) 3 , 2.57g EDTA is added to 14.83mL 85% H 3 PO 4 After stirring to dissolve, dilute with pure water to 51mL(nEDTA:nFe 3+ =1:1), mix well and pour into a glass reaction device, and finally add 9mL, 18mL and 27mL of freshly produced nanobubble water respectively, control the reaction temperature at 40℃, 45℃ and 50℃, after sealing, open the concentration Start to ventilate the 1000 ppm phosphine hydrogen cylinder, and adjust the gas flow rate to stabilize at 20 mL / min.
[0099] Such as Figure 5 As shown, as the amount of nanobubble water added increases, the initial dephosphorization rate of the complexed iron and jet combined system also increases. The maximum dephosphorization rate of the system can reach more than 50% at 27mL.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com