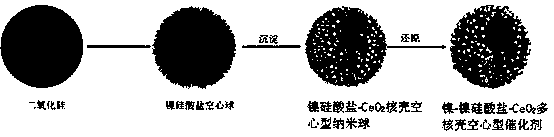
Method for preparing multi-nuclear-shell hollow nickel-nickel silicate-CeO2 through methane reforming
A hollow, silicate technology, used in chemical instruments and methods, catalyst activation/preparation, inorganic chemistry, etc., can solve problems such as loss of high specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) 200mL ethanol, 100mL water and 40mL orthosilicate methyl ester at 0 o Mix and stir evenly at C, then add urea to adjust the pH to 10. After stirring for 2h, it was separated with a centrifuge and washed with a mixture of methanol and water. Finally, 600nm silica nanoparticles were obtained at 150 o C dried for 24h.
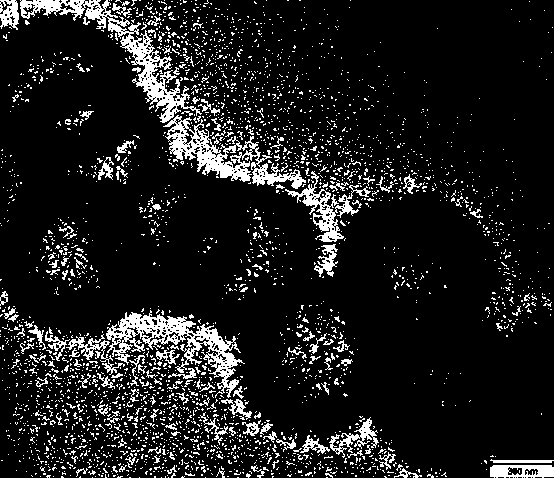
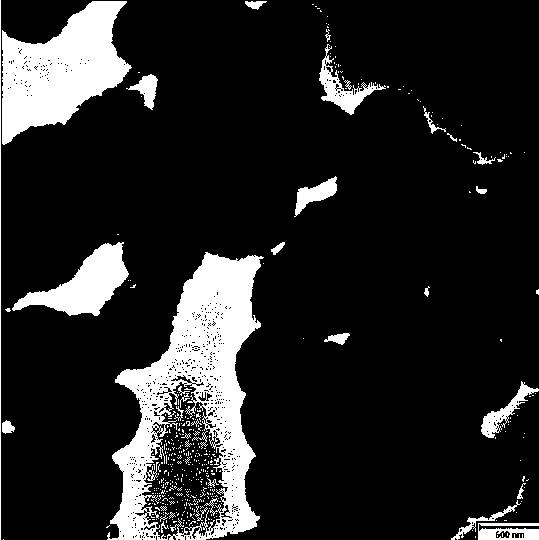
[0025] (2) Take 2g of silicon dioxide and 0.3g of nickel nitrate, add ammonia water, and adjust the pH to 8. Put the mixed solution into the autoclave and heat to 50 o C degree, after reacting for 24h, cool to room temperature. Centrifuge, wash with methanol, ethanol, and water, and place in a 100-degree drying oven. Obtain nickel silicate hollow spheres (such as figure 2 shown), the specific area is 250m 2 g -1 .
[0026] (3) Disperse hollow nickel silicate spheres in a mixed solution of ethanol (10mL) and water (20mL). After stirring for 30 min, cerium nitrate (3 g) and ammonia water (30 mL) were added. Raise the reaction temperature to 40...
Embodiment 2
[0030] (1) 200mL ethanol, 100mL water and 40mL methyl orthosilicate at 35 o C and mix well. Add urea to adjust the pH to 10. After stirring for 2h, it was separated with a centrifuge. Wash with a mixture of methanol and water. Finally, 600nm silica nanoparticles were obtained at 150 o C dried for 24h.
[0031] (2) Take 2g of silicon dioxide and 0.3g of nickel nitrate, add ammonia water, and adjust the pH to 11. Put the mixed solution into the autoclave and heat it to 135 o C degree, after reacting for 24h, cool to room temperature. Centrifuge and wash with methanol, ethanol, water, and then prevent from a 100-degree drying oven. Obtain nickel silicate hollow spheres (such as figure 2 shown), the specific area is 250m 2 g -1 .
[0032] (3) Disperse hollow nickel silicate spheres in a mixed solution of ethanol (10mL) and water (20mL). After stirring for 30 min, cerium nitrate (3 g) and ammonia water (30 mL) were added. Raise the reaction temperature to 95 o C, rea...
Embodiment 3
[0036] (1) 200mL ethanol, 100mL water and 40mL methyl orthosilicate at 70 o C and mix well. Add urea to adjust the pH to 10. After stirring for 2h, it was separated with a centrifuge, and washed with methanol and water. Finally, 600nm silica nanoparticles were obtained at 150 o C dried for 24h.
[0037] (2) Take 2g of silicon dioxide and 0.3g of nickel nitrate, add ammonia water, and adjust the pH to 13. Put the mixed solution into the autoclave and heat to 220 o C degree, after reacting for 24h, cool to room temperature. Centrifuge and wash with methanol, ethanol, water, and then prevent from a 100-degree drying oven. Obtain nickel silicate hollow spheres (such as figure 2 shown), the specific area is 250m 2 g -1 .
[0038] (3) Disperse hollow nickel silicate spheres in a mixed solution of ethanol (10mL) and water (20mL). After stirring for 30 min, cerium nitrate (3 g) and ammonia water (30 mL) were added. Raise the reaction temperature to 150 o C, reacted for 4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com