Duck plague live vaccine and preparation method thereof
A live and live vaccine technology for duck plague, applied in biochemical equipment and methods, vaccines, veterinary vaccines, etc., can solve problems such as the lack of DF duck plague virus method, the decline of live virus content in vaccines, and the impact on the use of vaccines, etc., to achieve The virus content and titer are not reduced, it is beneficial to storage and transportation, and the effect of heat resistance and protection is good
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 Preparation of duck plague virus liquid
[0056] After DF-1 cells were cultured to a single layer according to conventional methods, an inoculation group and a blank control group were established. The experimental group was inoculated with DPV F65 generation poison (the duck plague virus liquid DPV F65 generation poison that was weakened by CEF cells passaged) according to the volume ratio of 5% (ie 300ul), and the virus content was 10 7.5 TCID 50 / ml), at the same time, add 5% (ie 300ul) SPF chicken embryo allantoic fluid of 9-10 days old according to the volume of cell fluid 6ml, and place it at 37°C and 5% CO 2 Under the condition of adsorption for 60 minutes, the cell bottle was gently shaken every 15 minutes during the period, and the maintenance solution was added after the adsorption was completed. At the same time, the control group inoculated with CEF cells and DF-1 cells was set up as a blank control group. Viruses are the same.
[0057] Observe ...
Embodiment 2
[0060] Embodiment 2 Determination of virus content
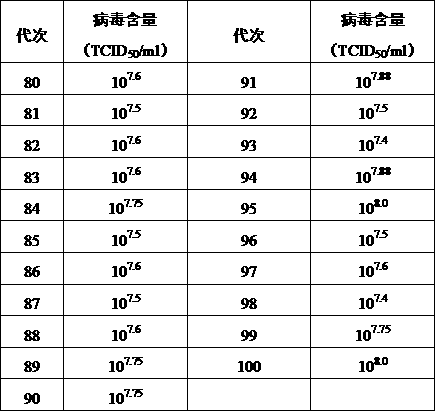
[0061] The DPV F80~F100 virus fluids of different generations harvested in Example 1 were serially diluted 10 times with serum-free M199, and 4 appropriate dilutions were taken to inoculate the 96-well micro-cell culture plate of CEF cells that had grown into a good monolayer. , each dilution was inoculated into 6 wells, 0.1ml per well, and a normal cell control was set at the same time. Set at 37°C, containing 5% CO 2 After adsorption in the incubator for 1 hour, add 0.1ml of M199 maintenance solution containing 4% serum to each well, observe for 120-144 hours, and record the number of cytopathic (CPE) wells. Calculate TCID by Reed-Muench method 50 , the results showed that the virus content of DPV F80~F100 generations was stable, and the virus content reached 10 7.4 ~10 8.0 TCID 50 / mL.
[0062] Table 1 Detection results of virus content
[0063]
Embodiment 3
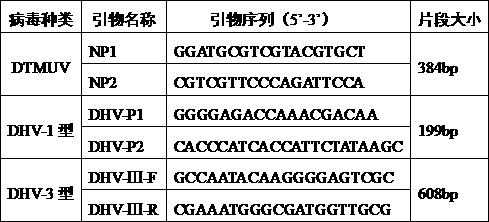
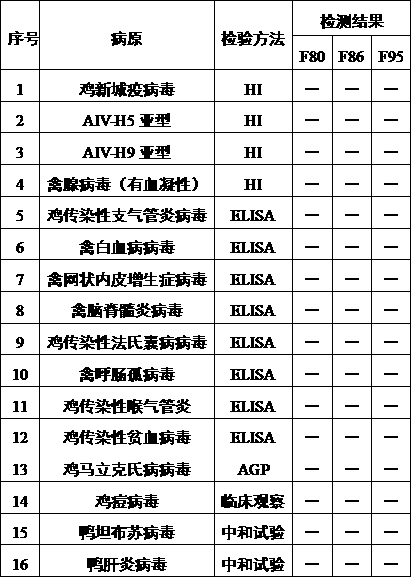
[0064] Example 3 Specific identification of DPV F80, F86, and F95 generations produced by DF-1 cells
[0065] The F80, F86, and F95 generation virus fluids harvested in Example 1 were diluted with serum-free M199 to contain 100 TCID 50 / 0.1ml, mixed with the same amount of anti-duck plague virus specific serum, neutralized at 37°C for 1 hour, inoculated into 6 cell wells (48-well cell plate) that had grown into a single layer of CEF, 0.2ml per well, and set There were 6 wells each for virus control and normal cell control. Set at 37°C, containing 5% CO 2 After culturing in an incubator and observing for 120-144 hours, the results showed that both the neutralization group and the normal cell control group had no cytopathic changes, and the cells in the virus control group showed cytopathic changes.
[0066] The preparation method of the anti-duck plague virus specific serum used in the present embodiment is:
[0067] (1) Preparation method of anti-duck plague virus specifi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com