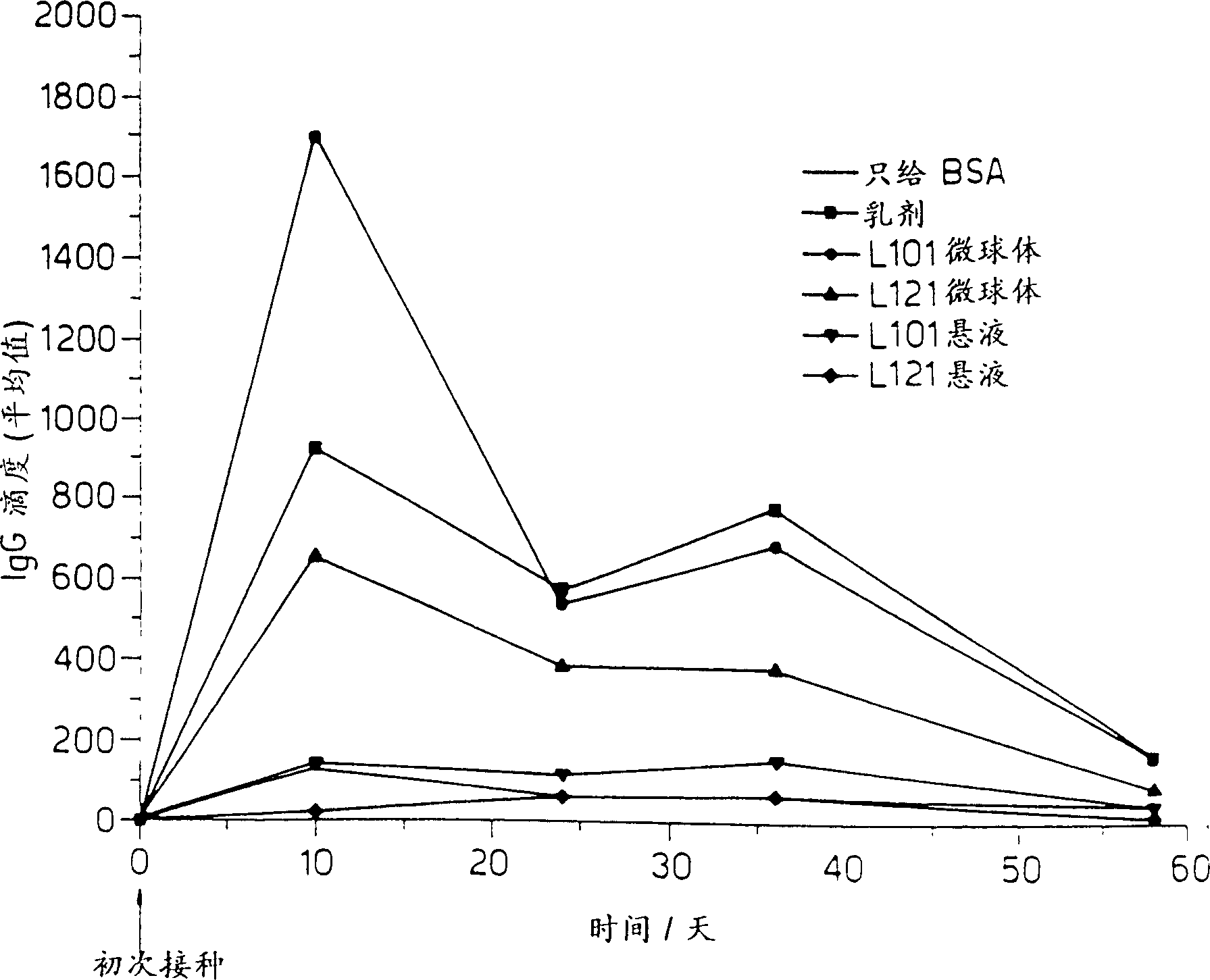
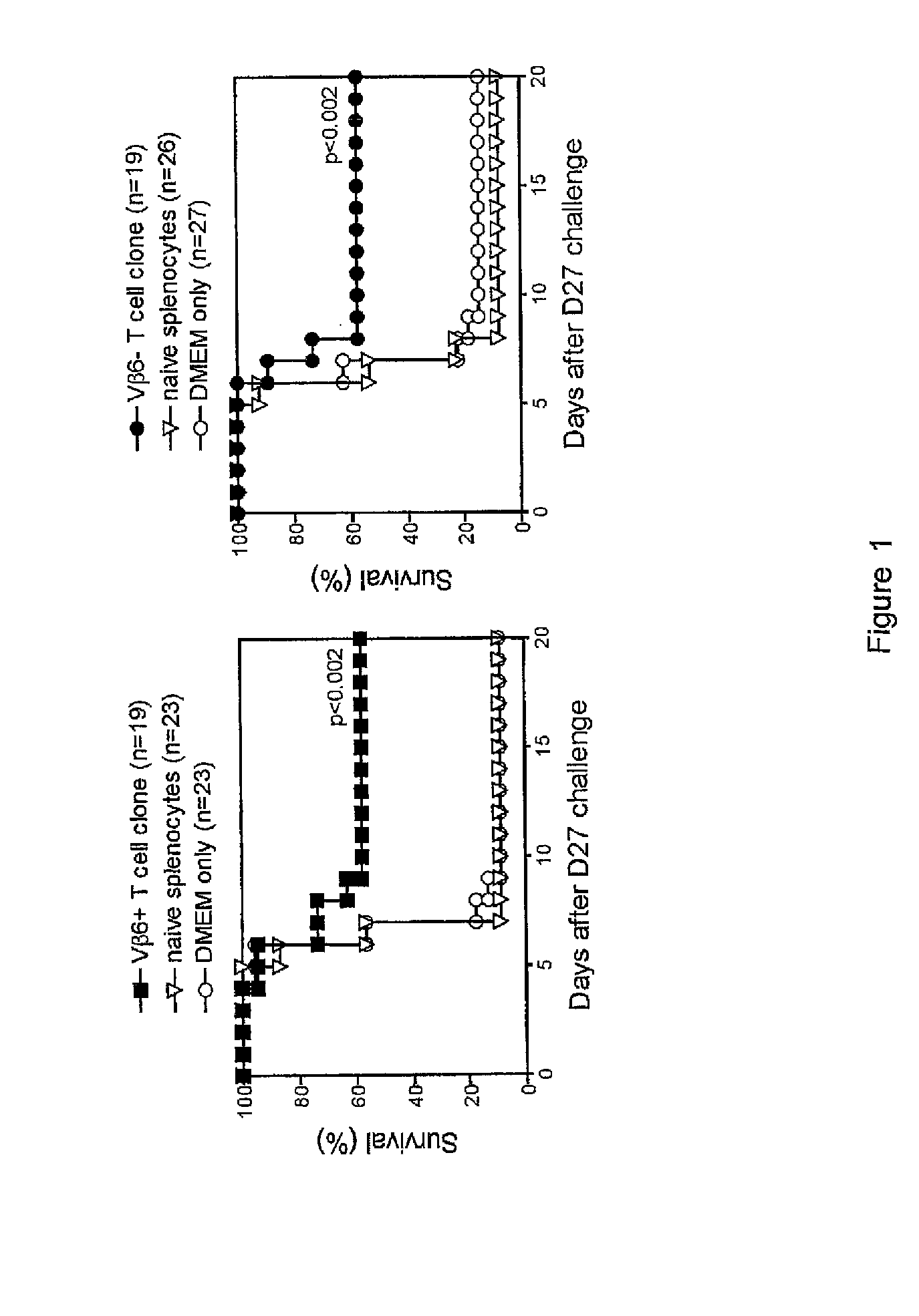
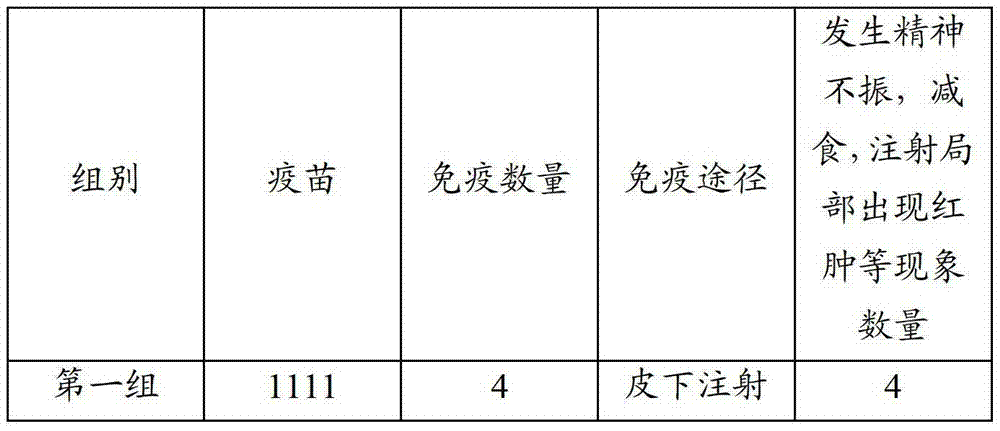
Patents
Literature
30 results about "Plague vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Plague vaccine is a vaccine used against Yersinia pestis. Killed bacteria have been used since 1890 but are less effective against pneumonic plague so that recently live vaccines of an attenuated type and recombination protein vaccines have been developed to prevent the disease.
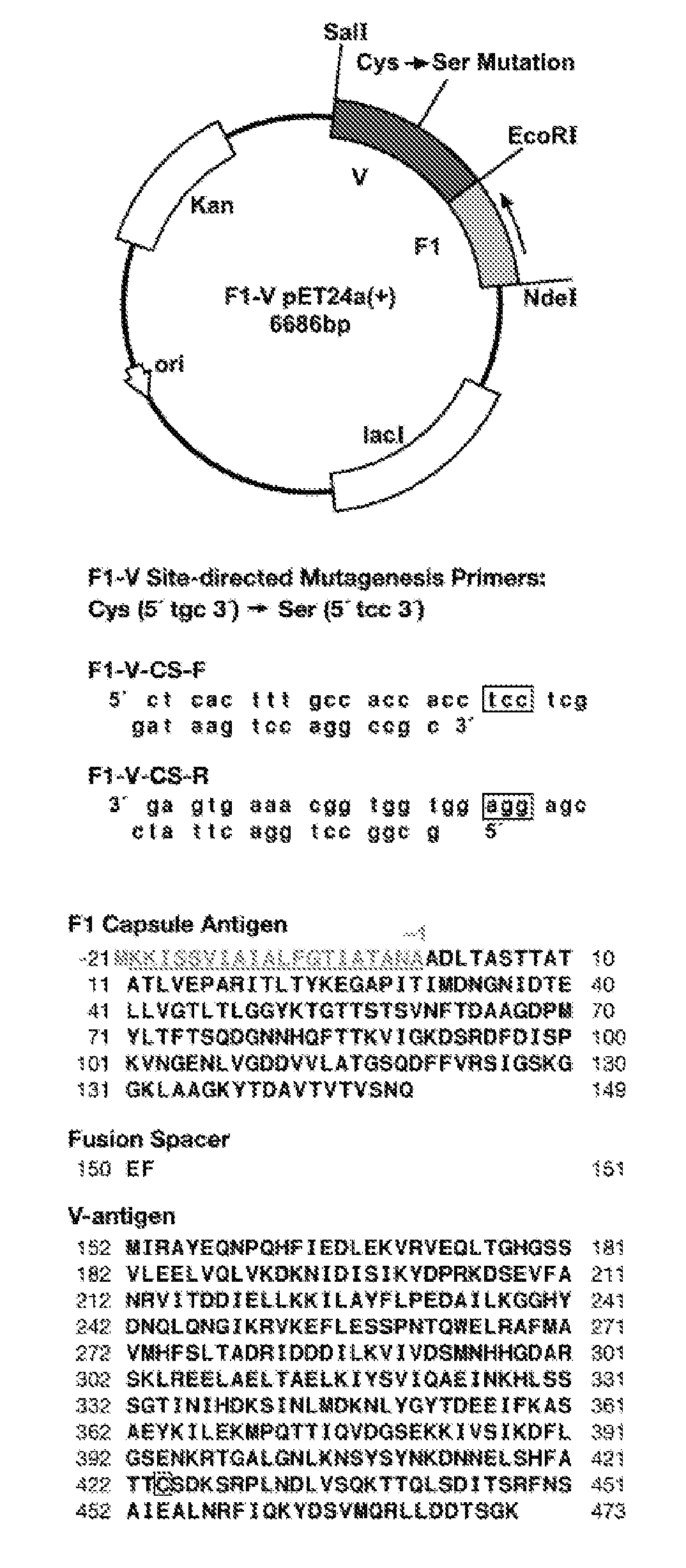
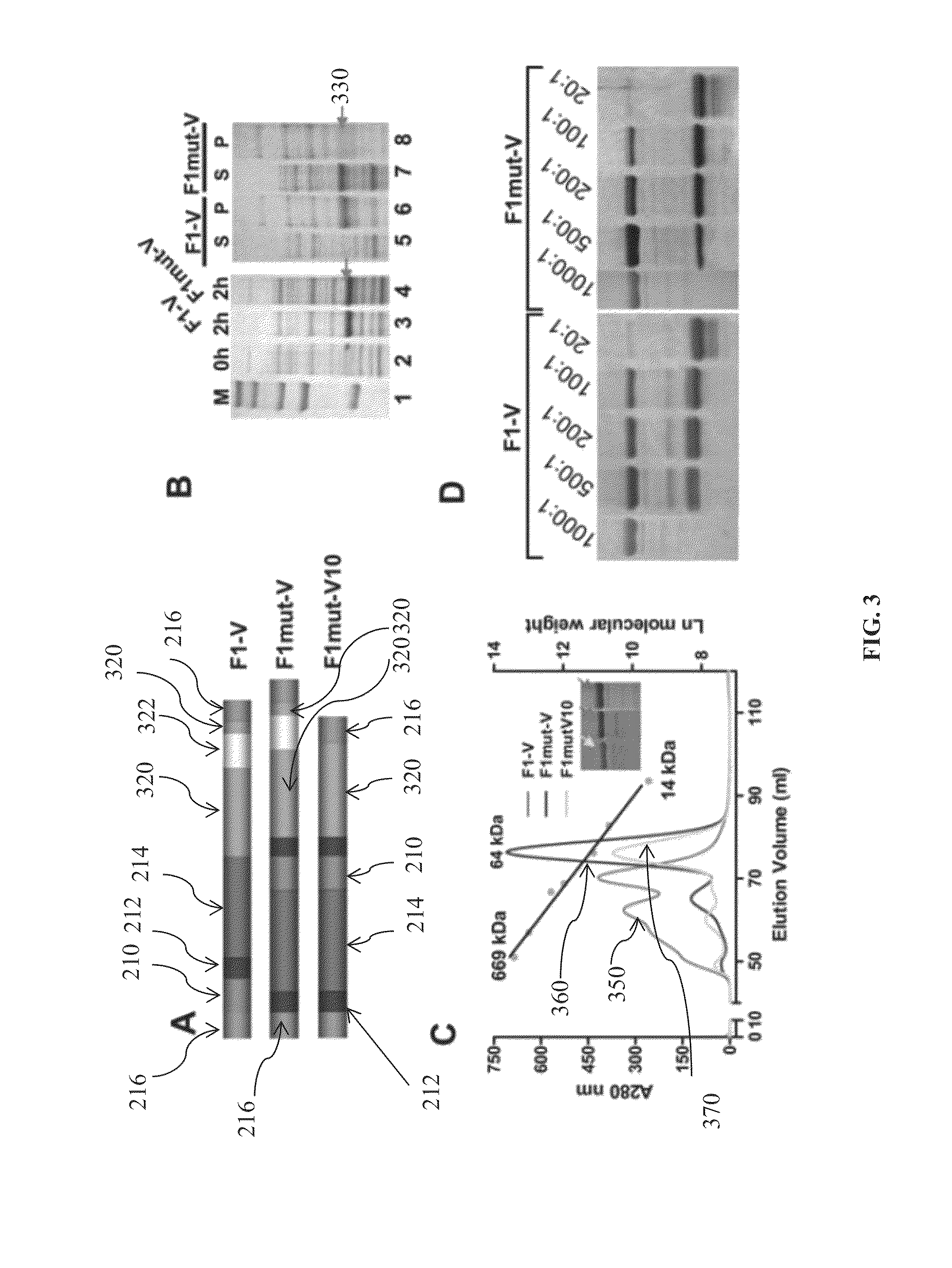
Purification and protective efficacy of monodisperse and modified yersinia pestis capsular f1-v antigen fusion proteins for vaccination against plague
InactiveUS20090130103A1Preserve immunogenicityAvoid infectionSugar derivativesAntibody mimetics/scaffoldsAntigenPlague vaccine
This disclosure concerns compositions and methods for the treatment and inhibition of infectious disease, particularly bubonic and pneumonic plague. In certain embodiments, the disclosure concerns immunogenic proteins, for instance substantially monodisperse F1-V fusion proteins, that are useful for inducing protective immunity against Y. pestis.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY DEPARTMENT OF HEALTH AND HUMAN SERVICES NATIONAL INSTITUTES OF HEALTH
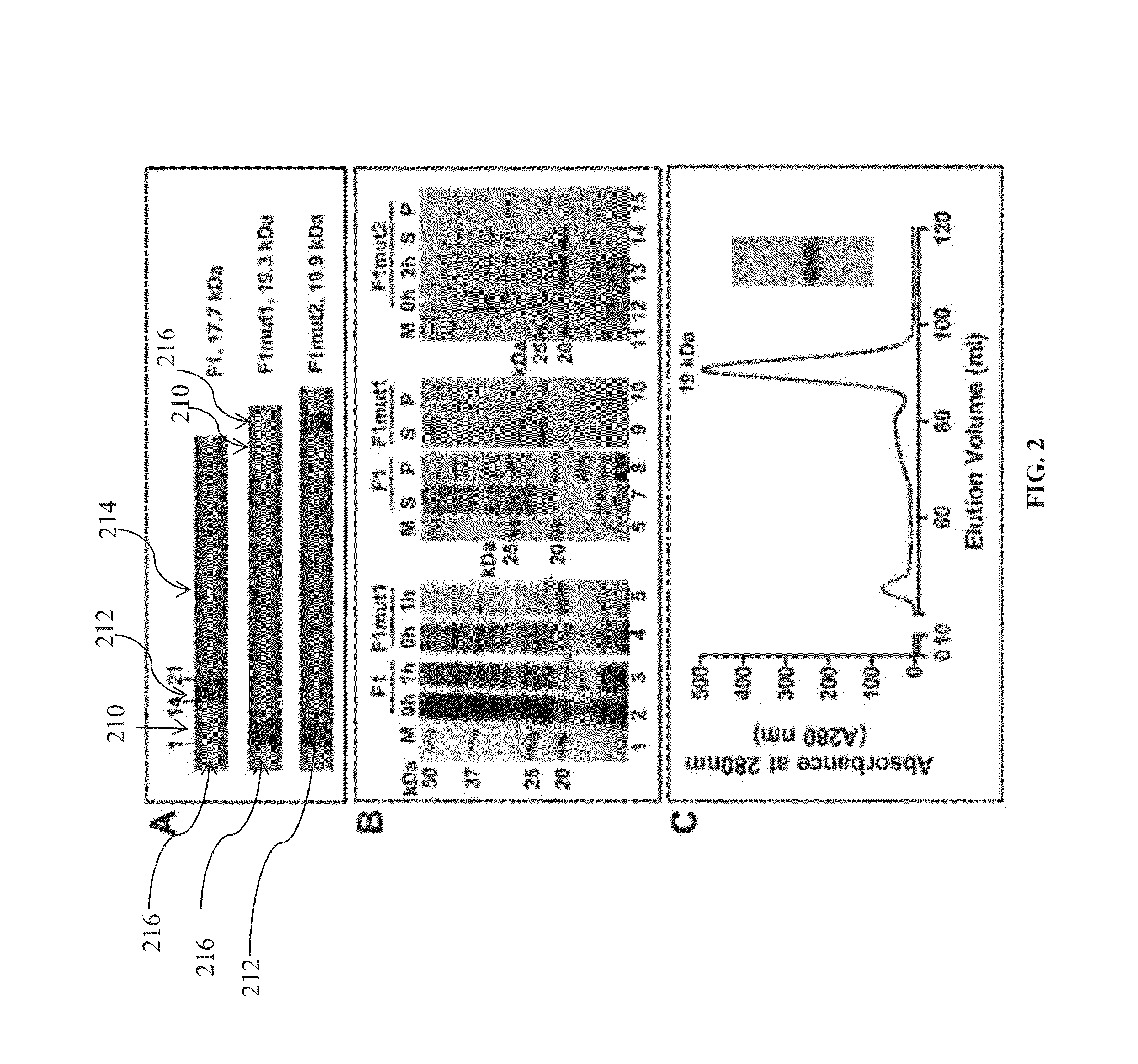
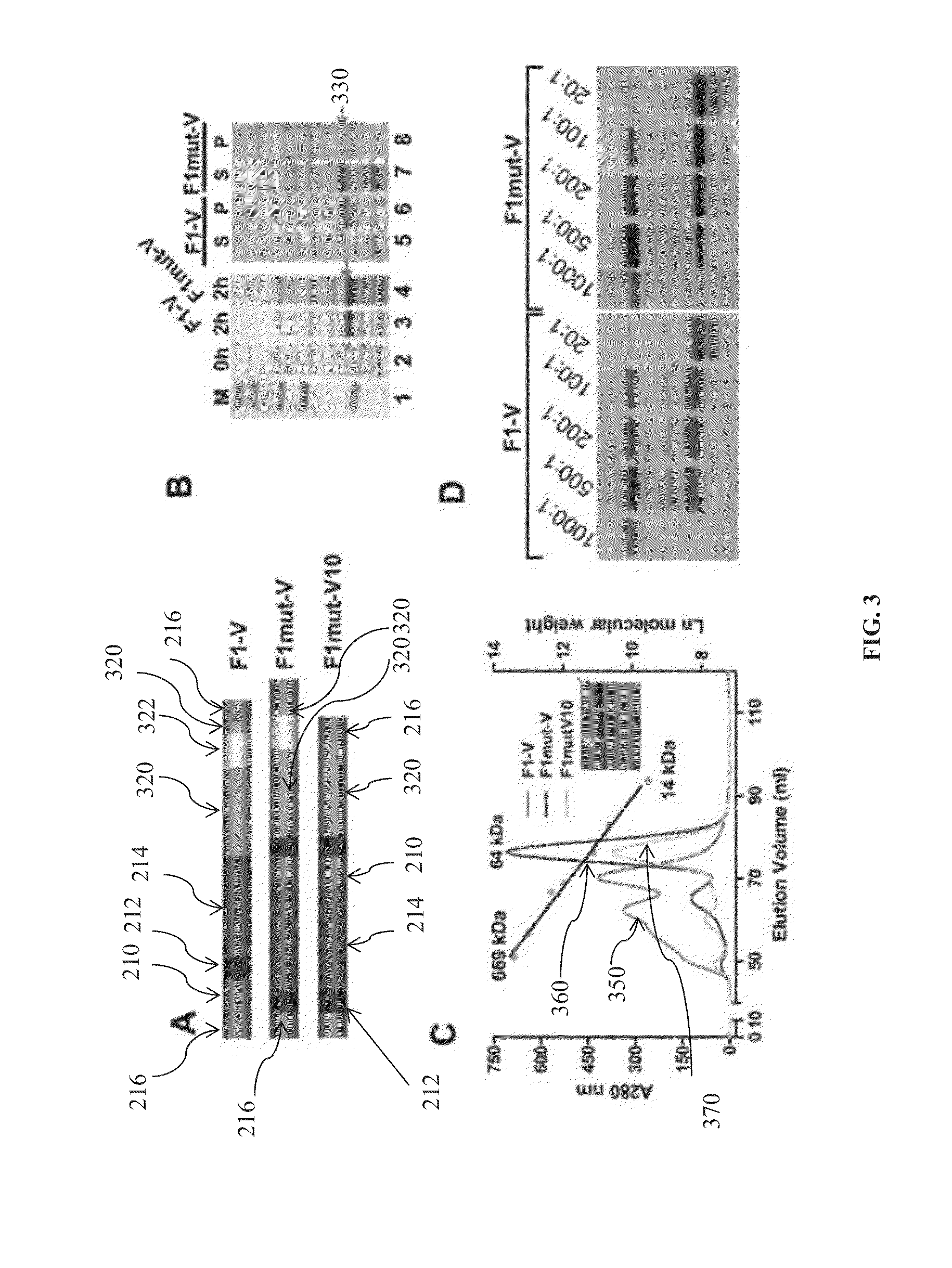
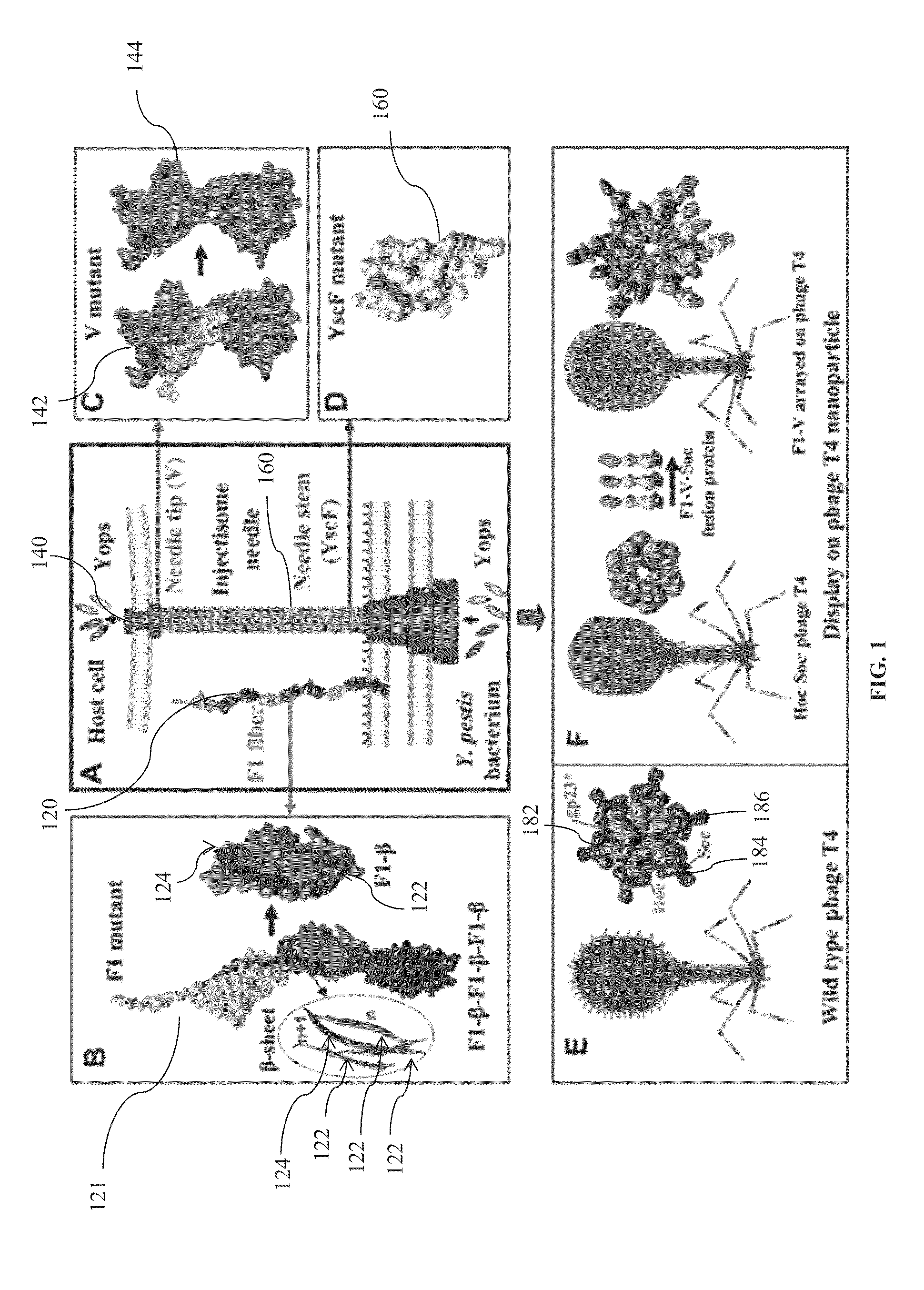
Mutated and bacteriophage t4 nanoparticle arrayed f1-v immunogens from yersinia pestis as next generation plague vaccines
Techniques from two basic approaches, structure-based immunogen design and phage T4 nanoparticle delivery, are developed to construct new plague vaccines. The NH2-terminal β-strand of F1 of Yersinia pestis is transplanted to the COOH-terminus of F1 of Yersinia pestis and the NH2-terminus sequence flanking the β-strand of F1 of Yersinia pestis is duplicated to eliminate polymerization but to retain the T cell epitopes. The mutated F1 is fused to the V antigen of Yersinia pestis to thereby form a fusion protein F1mut-V mutant, which produces a completely soluble monomer. The fusion protein F1mut-V is then arrayed on phage T4 nanoparticles via a small outer capsid protein, Soc, from a T4 phage or a T4-related phage. Both the soluble and T4 decorated F1mut-V provided approximately 100% protection to mice and rats against pneumonic plague evoked by high doses of Yersinia pestis CO92.
Owner:CATHOLIC UNIV OF AMERICA
Mutated and bacteriophage T4 nanoparticle arrayed F1-V immunogens from Yersinia pestis as next generation plague vaccines
Techniques from two basic approaches, structure-based immunogen design and phage T4 nanoparticle delivery, are developed to construct new plague vaccines. The NH2-terminal β-strand of F1 of Yersinia pestis is transplanted to the COOH-terminus of F1 of Yersinia pestis and the NH2-terminus sequence flanking the β-strand of F1 of Yersinia pestis is duplicated to eliminate polymerization but to retain the T cell epitopes. The mutated F1 is fused to the V antigen of Yersinia pestis to thereby form a fusion protein F1mut-V mutant, which produces a completely soluble monomer. The fusion protein F1mut-V is then arrayed on phage T4 nanoparticles via a small outer capsid protein, Soc, from a T4 phage or a T4-related phage. Both the soluble and T4 decorated F1mut-V provided approximately 100% protection to mice and rats against pneumonic plague evoked by high doses of Yersinia pestis CO92.
Owner:CATHOLIC UNIV OF AMERICA
Yersinia pestis virulence regulator TyrR and applications thereof
InactiveCN104789576AAntibacterial agentsBacterial antigen ingredientsPlague vaccineVirulent characteristics
A Yersinia pestis virulence regulator TyrR and applications thereof are provided. It is found that virulence of TyrR-deleted Yersinia pestis strains is lowered about 10000 times than virulence of wild strains, lack of the TyrR has no influence on in-vitro growth of the Yersinia pestis, and the TyrR can achieve negative regulation of transcription of five aromatic amino acid metabolism genes comprising aroF-tyrA, aroP, aroL and tyrP, and achieve positive regulation of two acid response genes which are hdeB and hdeD, so that the TyrR is proved to be a Yersinia pestis virulence regulator. In an environment having a pH value of 5.0, the survival rate and a growth speed of a Yersinia pestis TyrR mutant strain are lower than that of a wild strain, thus proving that the acid resistance of a TyrR mutant strain is lowered and that the TyrR regulator is closely related to acid resistance of the Yersinia pestis. Applications of the Yersinia pestis virulence regulator TyrR in preparation of anti-plague vaccines, and applications of the virulence regulator TyrR in preparation of medicines adopting the TyrR as a target are provided.
Owner:NANHUA UNIV
Protective antigen composition capable of excitating organism to generate immunity against plague and use thereof
The invention discloses a protective antigen composition which can motivate an organism to generate immunity to plague, and the application thereof in preparing a plague vaccine. The protective antigen composition is a composition which is mixed by a natural F1 antigen and an rV270 antigen according to the weight ration of 1-2 to 2-1. The immune protection effect of the composition is evaluated by animal experiments and the evaluation result shows that the protective antigen composition that is composed of the natural F1 antigen and the rV270 antigen performs good immune protection effect to the infection caused to mice, guinea pigs and rabbits by plague bacillus, has relatively high safety and can be used for preparing a subunit plague vaccine.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Ecological breeding method for beef cattle
InactiveCN107318781ARich in nutrientsEnhance physical fitnessFood processingAnimal feeding stuffNutritive valuesPlague vaccine
Provided is an ecological breeding method for beef cattle. The method includes the following steps of selecting a small hill with good air, shade and less wind; arranging ventilation and heating facilities in a cattle barn, and arranging a shower area; injecting plague vaccine for 30 - 40 days of beef cattle pups, and injecting combined vaccine against the plague and erysipelas lung diseases for 60 - 70 days of beef cattle pups; in the beef cattle fattening stage, stocking in the morning, breeding in the barn in the afternoon, putting cattle into the shower area to conduct shower cleaning every 3-5 days, keeping ventilation in the cattle barn, maintaining 22-26 DEG C of temperature at daytime, and maintaining 14-21 DEG C of temperature at night. The ecological breeding method for beef cattle is scientific and reasonable, combines the advantages of stocking and barn breeding, starts from the growth characteristics of the cattle, provides the cattle with reasonable nutrients, ensures proper movement of the cattle, and thus enhances the physique of beef cattle. The bred beef cattle are strong, the quality of the beef is guaranteed well, and the produced beef has the advantages of being rich in nutrient, good in taste and high in nutritive value, and meets people's demands for beef nutrition better.
Owner:道真自治县利达循环生态良种肉牛养殖有限公司
Murine plague mycoprotein with immunity protection function, encoding gene and uses thereof
InactiveCN101245100AImprove protectionExpand the scope ofAntibacterial agentsOrganic active ingredientsAntigenMycoprotein
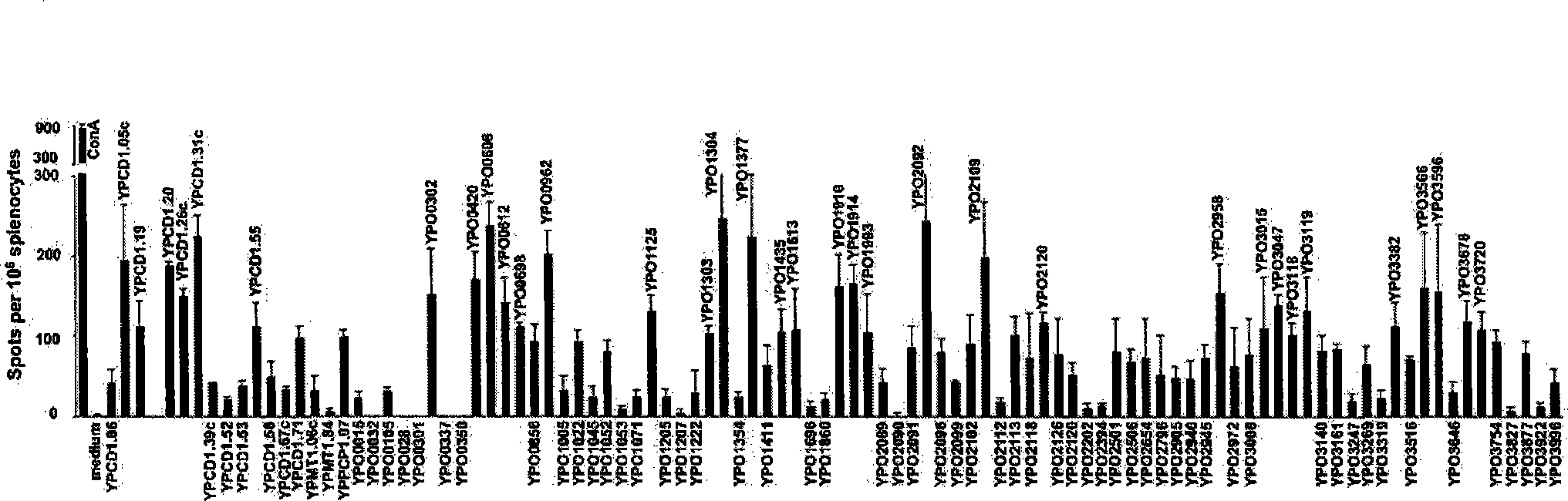
The invention discloses a plague bacteria protein with cellular immune protection function, an encoding gene and an application. The purpose is to provide the protein and the encoding gene which can stimulate T cell immune reaction during plague bacteria infection and immune processes and can generate cellular immune protection function, as well as the application in the preparation of plague vaccines. The experiments prove that the protein of the invention can stimulate T cell immune reaction during the infection and the immune processes of plague bacteria and can generate immune protection function. The protein or gene of the invention can be taken as an active ingredient for preparing the plague vaccines, and can enhance the immune protection effect and the immune protection region of the existing plague vaccines through mutual immunization or the combined immunization with the existing F1 and LcrV antigen or gene with immune protection function, or the preparation of a recombinant fusion multivalent subunit vaccine or DNA vaccine at the same time. The invention provides a new means for searching the protein of the plague bacteria with cellular immune protection function, which can play the important role in the prevention and the treatment of plague and have broad application prospect.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Weaned young rabbit breeding method
InactiveCN102783453AEnsure normal growth and developmentImprove survival rateAnimal husbandryPlague vaccineFishery
The invention provides a weaned young rabbit breeding method. The weaned young rabbit breeding method comprises the following steps: adding 5% milk powder into feed for a weaned young rabbit segmentally; adding a rabbit vibrio eliminating medicine into water or the feed to prevent enteritis of the young rabbit; injecting rabbit plague vaccine to prevent rabbit plague of the young rabbit; and adding sulfamide coccidian and diclazuril into the feed or water to prevent and control coccidiosis of the young rabbit. According to the weaned young rabbit breeding method, through comprehensive utilization of determination on a young rabbit weaning standard, feed control and management, and segmental prevention of the enteritis, the rabbit plague and the coccidiosis, the normal growth and development of the weaned young rabbit can be ensured, the survival rate of the young rabbits is increased from 65% to 95%, the young rabbits grow rapidly and can be slaughtered earlier, and the meat quality and the fur quality are high; therefore, the weaned young rabbit breeding method is applicable to breeding rabbits.
Owner:刘文广
Test paper bar for testing colloidal gold of F1 antibody of plague bacterium
This invention provides one rapid test bar for plague vaccine, which covers plague vaccine F1 antigen and plague antibiosis F1 multiple clones antigens on the NC film and combines glue gold label antigen and applies film analysis double antigen clamper method and tests human and animal blood specimen plague antibiosis level.
Owner:BEIJING ZHUANGDI HAOHE BIOMEDICINE SCI & TECH
Vaccines for plague
A method of protecting a human or animal body from the effects of infection with Y. pestis is provided comprising administering to the body a vaccine including Yersinia pestis V antigen and Yersinia pestis F1 antigens or a protective epitopic part of each of these in a form other than whole Y. Pestis organisms. Preferably the antigens are administered in the form of a live vaccine or as recombinantly produced isolated and / or purified proteins. DNA encoding the whole or part of the F1 antigen and DNA encoding the whole or part of the V antigen may be used directly as a genetic vaccine.
Owner:THE SEC OF STATE FOR DEFENCE IN HER BRITANNIC MAJESTYS GOVERNMENT OF THE UK OF GREAT BRITAIN & NORTHERN IRELAND
Uses of yersinia yope peptide, gene and subparts thereof as a plague vaccine component and assays for yersinia pestis-specific t cells
InactiveUS20130273092A1Rapid responseRespond faster and moreBacterial antigen ingredientsBiological material analysisPlague vaccinePeptide antigen
Owner:TRUDEAU INST INC
Recombinant F1-V Plague Vaccine
InactiveUS20120164156A1Good curative effectSaving can be considerableAntibacterial agentsBacterial antigen ingredientsPlague vaccineYersinia enterocolitica
Disclosed herein is a method of inducing an immunological response in a subject which comprises administering an immunogenic amount of a purified fusion protein comprising all or part of F1 antigen of Yersinia pestis fused to the amino terminus of all of V antigen of Yersinia pestis, Yersinia enterocolitica, or Yersinia pseudotuberculosis to the subject.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Recombinant F1-V Plague Vaccine
InactiveUS20090022756A1Good curative effectSaving can be considerableAntibacterial agentsBacterial antigen ingredientsPlague vaccineAntigen
Disclosed herein is a composition comprising a purified fusion protein comprising all or part of F1 antigen of Yersinia pestis fused to the amino terminus of all of V antigen of Yersinia pestis, Yersinia enterocolitica, or Yersinia pseudotuberculosis that is isolated from its expression vector.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Compound plague vaccine and preparation method thereof
InactiveCN101337069AAvoid or reduce side effectsImprove securityAntibacterial agentsBacterial antigen ingredientsEscherichia coliAdjuvant
The invention provides a composite plague vaccine and the preparation method thereof. The plague vaccine comprises thallus split products of the attenuated strain of plague bacilli and recombination plague bacilli V antigens, and further comprises plague bacilli F1 antigens, wherein, by collecting the thallus of plague attenuated strain grown on the culture medium, the thallus split products of the plague attenuated strain is obtained through the cracking preparation by high-pressure homogenization. Bacterial cell wall with good adjuvant effect, bacterial protein with antigenic specificity (which includes protective capsular antigen F1) and CpG fragments which are rich in bacterial DNA, with stronger immunological competence, are concentrated in the split products; the expression vector including plague bacilli V antigens or F1 antigens which are constructed by adopting the gene recombination technology is transformed into escherichia coli, and after the inducible expression is performed, recombination plague bacilli V antigens or F1 antigens are obtained through purification. The vaccine is applicable to the general population for immunizing and preventing the plague.
Owner:NAT INST FOR THE CONTROL OF PHARMA & BIOLOGICAL PROD
Gosling plague viral vaccine
ActiveCN106075426AImprove securityAvoid infectionViral antigen ingredientsAntiviralsMaternal antibodyViral Vaccine
The invention aims at providing a muscovy-duck-source gosling plague virus inactivated vaccine. The vaccine comprises an antigen and a vaccine adjuvant. The adopted antigen is an inactivated muscovy-duck-source gosling plague virus, and the preservation number of the adopted muscovy-duck-source gosling plague virus is CCTCC NO:V201620. A muscovy-duck-source gosling plague virus YBGPV-M strain with low toxicity and high immunogenicity is screened out and then inoculated with a duck embryo, the infected embryo and allantoic fluid are collected, after homogenization, ultrafiltration and concentration and formaldehyde solution inactivation, the oil adjuvant is added, and the vaccine is prepared after mixing and emulsifying. The prepared vaccine immunizes breeding muscovy ducks, the antibody level of the breeding muscovy ducks can be improved, the offspring maternal antibody level of the breeding muscovy ducks is guaranteed, and young muscovy duck gosling plague virus infection caused by the muscovy-duck-source gosling plague virus is prevented; antibodies can be produced after 10 days when 1-day young muscovy ducks are immunized, and young muscovy duck gosling plague virus infection caused by the muscovy-duck-source gosling plague virus can be effectively prevented. The prepared vaccine has the advantages of being efficient and good in safety.
Owner:YEBIO BIOENG OF QINGDAO
A Streptococcus equine epidemic strain xz1 and its application in equine epidemic vaccine
ActiveCN103421708BReduce manufacturing costImprove product added valueAntibacterial agentsBacterial antigen ingredientsHemolysisMannitol
The invention relates to a strain of strangles streptococcus (also known as streptococcus. equi subsp equi) XZ1 with the preservation number of CGMCC No.7830. The strangles streptococcus strain has the16S rRNA gene sequence shown in the sequence table 1; the strain is isolated from sick horses with strangles and has gram positive bacteria, and cells are spherical and are arranged in pairs or cells are bent chain-shaped, which are in poor growth in ordinary broth and plate; beta type hemolysis happens on a plate with 5% of sheep blood, and a completely transparent hemolytic tape with the width of 3 mm can be formed around small dewdrop-shaped bacterial colonies; facultative anaerobic growth can be realized with the growth temperature from 25 DEG C to 40 DEG C, and the optimum growth temperature is 37 DEG C, and the optimum pH value for growth is between 7.4 and 7.5; glucose is fermented for production of acid, and lactose, mannitol and sorbitol cannot be fermented. The strangles streptococcus strain can be used for preparation of strangles inactivated vaccine, and the prepared strangles inactivated vaccine is good in pertinence, low in cost, safe and good in protection effect.
Owner:XINJIANG AGRI UNIV
Plague vaccine
ActiveCN104781391AStable genomeGuaranteed responsePeptidesAntibody medical ingredientsPlague vaccineMicrobiology
The application relates to a Yersinia pseudotuberculosis cell, which comprises nucleic acid coding for expression of at least one Yersinia pestis Caf1 polypeptide or of at least one antigenic fragment of Yersinia pestis Caf1, more particularly to an attenuated Y. pseudotuberculosis cell, which expresses the Y. pestis capsule. Said Y. pseudotuberculosis cell is exceptionally efficient in protecting against both bubonic plague and pulmonary plague.
Owner:INST PASTEUR
Method for removing lipoprotein and lipid in goose/duck yolk by using freezing method
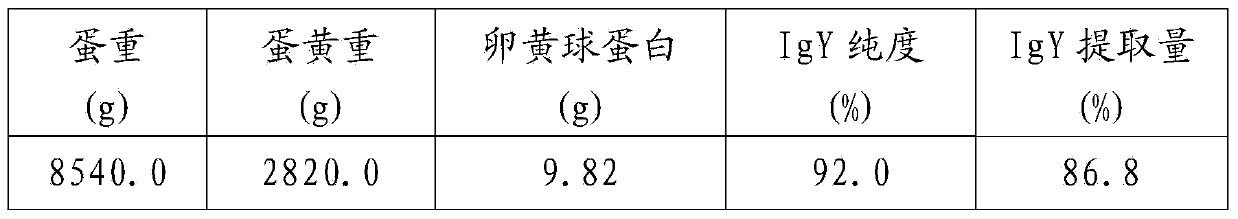
The invention discloses a method for removing lipoprotein and lipid in goose / duck yolk by using a freezing method. The method comprises the following steps of (1) cleaning and disinfecting a fresh egg laid by a goose / duck supplementarily immunized by using a goose vaccine; (2) separating egg white from yolk; (3) extracting 1 part of separated yolk according to a certain volume, adding 3 times of distilled water with the same volume to dilute, uniformly stirring, and storing under freezing at the temperature of 18 DEG C below zero for 24h and longer to ensure that the yolk is solidly frozen; (4) taking out the solidly frozen yolk, unfreezing, and filtering to obtain colorless and transparent filtrate capable of purifying immunoglobulins IgY. The goose vaccine in the step (1) is a gosling plague vaccine, a goose paramyxovirus vaccine or an avian influenza vaccine. By using a physical freezing separation method, a yolk sediment is sufficiently utilized, and no chemical organic solvents are adopted, so that the cost can be remarkably reduced.
Owner:ANHUI SCI & TECH UNIV
Live attenuated vaccine strain of plague bacillus and application thereof as lung delivery vaccine
InactiveCN110129250AImprove survival rateAntibacterial agentsBacterial antigen ingredientsPlague bacillusNucleotide
The invention discloses a live attenuated vaccine strain of plague bacillus and an application thereof as a lung delivery vaccine. The invention discloses plague bacillus EV-B-SHV[delta]pla; comparedwith a genomic DNA of plague bacillus EV-B-SHV, the plague bacillus EV-B-SHV[delta]pla has the difference only in that a DNA molecule shown in nucleotide at 7th-906th site of a DNA molecule shown in asequence 1 of a sequence table is knocked out. The invention also discloses an application of the plague bacillus EV-B-SHV[delta]pla in preparation of a plague vaccine. The plague vaccine is given bya lung delivery way. The invention also discloses the plague vaccine; the active ingredient of the plague vaccine is the plague bacillus EV-B-SHV[delta]pla. The detection results of specific antibodies, cytokines and the bacterial load amount show that the plague bacillus EV-B-SHV[delta]pla can be used as a vaccine strain for immunization inoculation by the lung delivery way.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Yersinia pestis protein and coding gene with immune protection function and its application
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Breeding method for black swans in southern region of China
InactiveCN108244028AHair color is black and brightLess sicknessFood processingAnimal feeding stuffFree rangeParamyxovirus vaccine
The invention discloses a breeding method for black swans in the southern region of China. The method comprises the following steps: a stocking water area is provided for the black swans according toan area of 65-75m<2> of each pair of black swans, and at least one place with trees or shrubs exist near the water area; the water area is disinfected once every three months; in the gosling period, feeding is performed for three times a day by using first concentrated feed, feeding is performed once a day by using green feed, and water is sufficient, in the young goose and adult goose periods, feeding is performed twice a day by using first type feed, and in the egg producing stage of breeding geese, feeding is performed four times a day by using second concentrated feed, and green feed is freely supplied; a gosling plague vaccine is injected within one week to half a month after goslings are born, and a goose paramyxovirus vaccine is injected from March 15 to April 15 every year; and enough nesting materials are provided near the water area, after the black swans build a nest freely, a shack is built at the top of the nest, and cleanliness and hygiene near the shack are guaranteed. According to the method provided by the invention, in addition to partial artificial intervention in the gosling period, the bred black swans are mainly free-range, and the bred black swans have a black and bright hair color, a healthy body posture, less illness and a greater ornamental value.
Owner:CHONGQING LINGFENG TECH
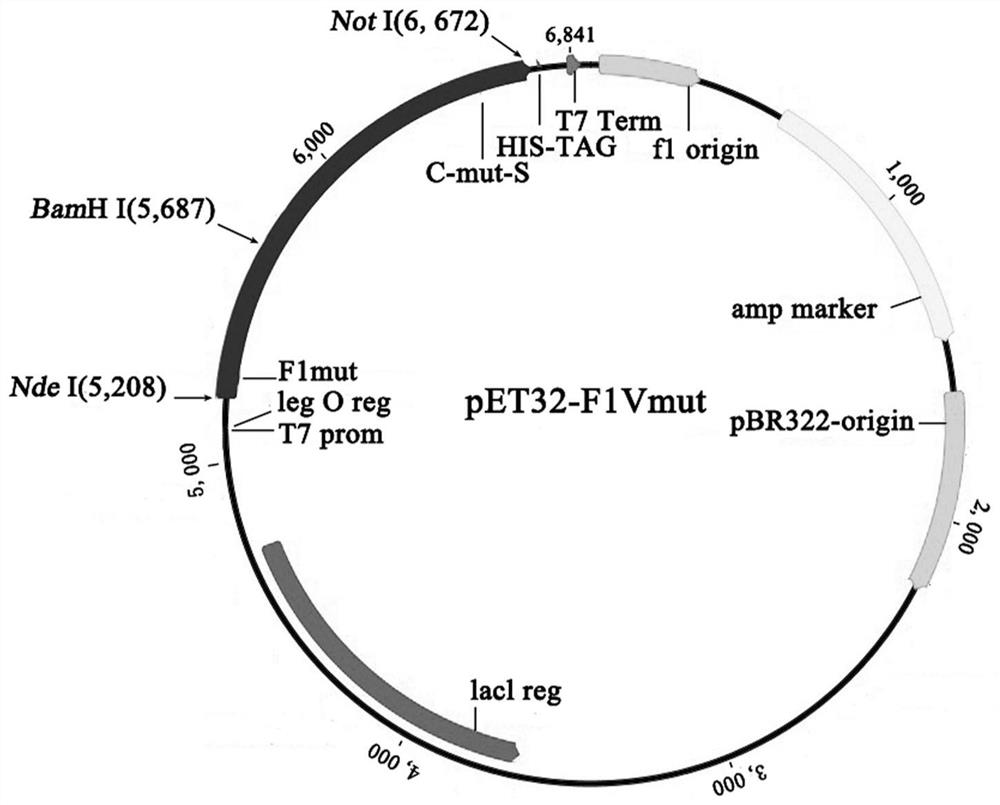
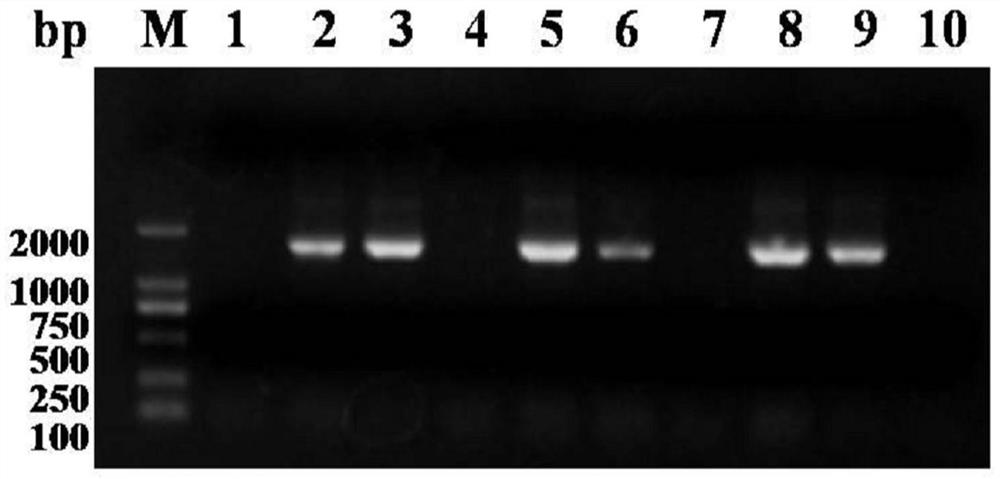
A kind of purification method of Yersinia pestis f1vmut fusion protein
ActiveCN108070036BSimple purification methodFast purification methodAntibody mimetics/scaffoldsPeptide preparation methodsUltrafiltrationAnion-exchange chromatography
The invention discloses a yersinia pestis F1Vmut fusion protein purification method. The method includes four steps including bacterial lysis, anion exchange chromatography, hydrophobic interaction chromatography and ultrafiltration. According to a purification process, more than 80mg of antigens in purity higher than 95% can be obtained by consumption of each liter of culture only through two-step column chromatography and stepwise elution, and the antigens are less prone to aggregation and beneficial to product quality control. By adoption of the method, high target protein yield and linearrepeatability are realized, industrial amplification is benefited, and the recombinant antigens excellent in immunogenicity and protectiveness are finally obtained. In addition, by adoption of the method, recombinant F1Vmut large-scale preparation can be realized, and a great foundation is laid for industrialization of recombinant plague vaccines.
Owner:ACADEMY OF MILITARY MEDICAL SCI
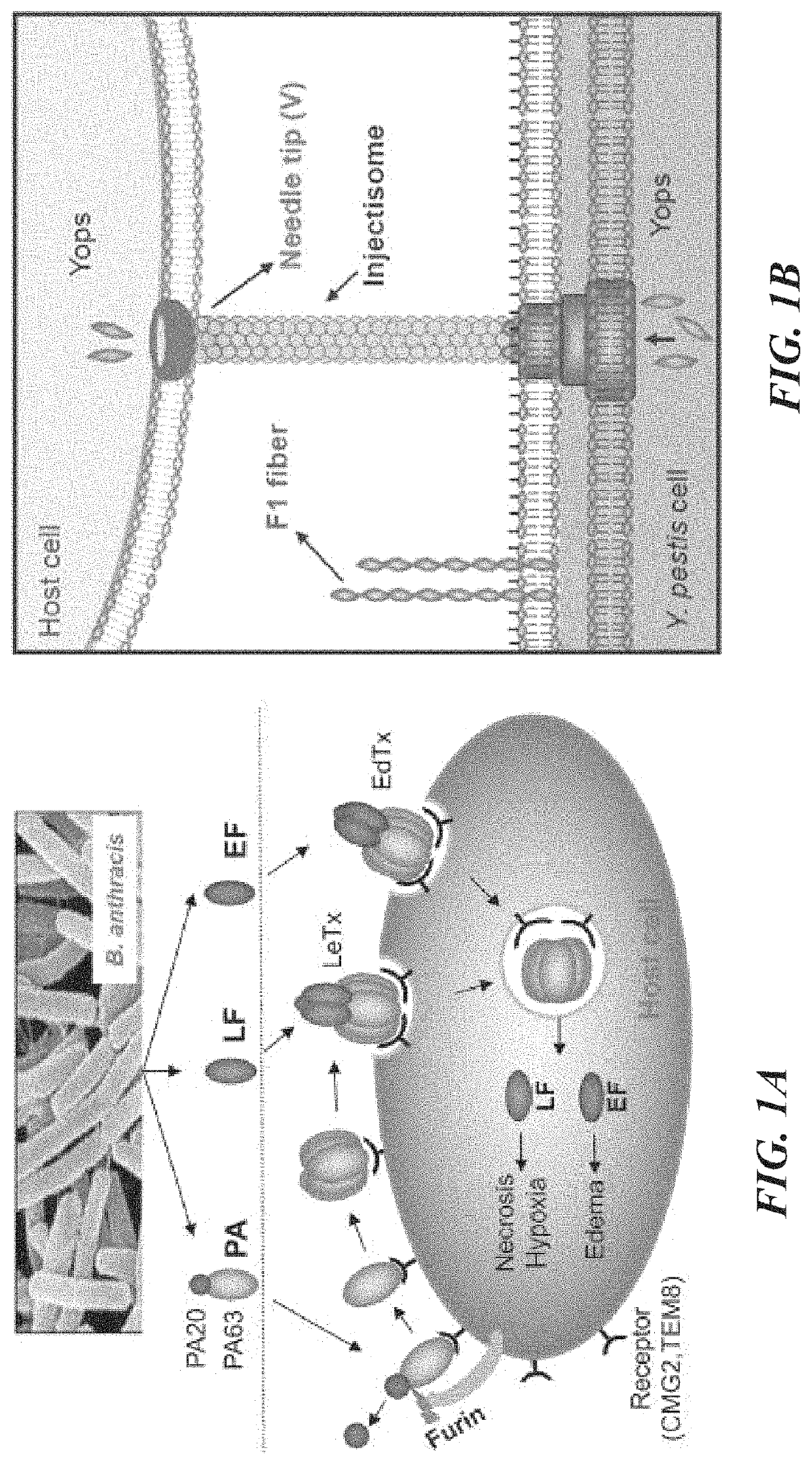
Dual anthrax-plague vaccines that can protect against two tier-1 bioterror pathogens, bacillus anthracis and yersinia pestis
ActiveUS20180264102A1Bacterial antigen ingredientsAntibody mimetics/scaffoldsPlague vaccineAnaplasma phagocytophilum
Bivalent immunogenic compositions against anthrax and plague are disclosed herein. One bivalent immunogenic composition comprises a triple fusion protein containing three antigens, F1 and V from Yersinia pestis and PA antigen from Bacillus anthracia fused in-frame and retaining structural and functional integrity of all three antigens. Another bivalent immunogenic composition comprises bacteriophage nanoparticles arrayed with these three antigens on the capsid surface of the bacteriophage nanoparticles. These bivalent immunogenic compositions are able to elicit robust immune response in a subject administered said the bivalent immunogenic compositions and provide protection to the subject against sequential or simultaneous challenge with both anthrax and plague pathogens.
Owner:CATHOLIC UNIV OF AMERICA
Yersinia pestis virulence regulator tyrr and its application
A Yersinia pestis virulence regulator TyrR and applications thereof are provided. It is found that virulence of TyrR-deleted Yersinia pestis strains is lowered about 10000 times than virulence of wild strains, lack of the TyrR has no influence on in-vitro growth of the Yersinia pestis, and the TyrR can achieve negative regulation of transcription of five aromatic amino acid metabolism genes comprising aroF-tyrA, aroP, aroL and tyrP, and achieve positive regulation of two acid response genes which are hdeB and hdeD, so that the TyrR is proved to be a Yersinia pestis virulence regulator. In an environment having a pH value of 5.0, the survival rate and a growth speed of a Yersinia pestis TyrR mutant strain are lower than that of a wild strain, thus proving that the acid resistance of a TyrR mutant strain is lowered and that the TyrR regulator is closely related to acid resistance of the Yersinia pestis. Applications of the Yersinia pestis virulence regulator TyrR in preparation of anti-plague vaccines, and applications of the virulence regulator TyrR in preparation of medicines adopting the TyrR as a target are provided.
Owner:NANHUA UNIV
Plague vaccine
ActiveUS20150202275A1High protection levelFacilitates vaccinationBacterial antigen ingredientsPeptidesPlague vaccineMicrobiology
The application relates to a Yersinia pseudotuberculosis cell, which comprises nucleic acid coding for expression of at least one Yersinia pestis Caf1 polypeptide or of at least one antigenic fragment of Yersinia pestis Caf1, more particularly to an attenuated Y. pseudotuberculosis cell, which expresses the Y. pestis capsule. Said Y. pseudotuberculosis cell is exceptionally efficient in protecting against both bubonic plague and pulmonary plague.
Owner:INST PASTEUR
Dual anthrax-plague vaccines that can protect against two tier-1 bioterror pathogens, Bacillus anthracis and Yersinia pestis
Bivalent immunogenic compositions against anthrax and plague are disclosed herein. One bivalent immunogenic composition comprises a triple fusion protein containing three antigens, F1 and V from Yersinia pestis and PA antigen from Bacillus anthracia fused in-frame and retaining structural and functional integrity of all three antigens. Another bivalent immunogenic composition comprises bacteriophage nanoparticles arrayed with these three antigens on the capsid surface of the bacteriophage nanoparticles. These bivalent immunogenic compositions are able to elicit robust immune response in a subject administered said the bivalent immunogenic compositions and provide protection to the subject against sequential or simultaneous challenge with both anthrax and plague pathogens.
Owner:CATHOLIC UNIV OF AMERICA
Compound plague vaccine and preparation method thereof
The invention provides a composite plague vaccine and the preparation method thereof. The plague vaccine comprises thallus split products of the attenuated strain of plague bacilli and recombination plague bacilli V antigens, and further comprises plague bacilli F1 antigens, wherein, by collecting the thallus of plague attenuated strain grown on the culture medium, the thallus split products of the plague attenuated strain is obtained through the cracking preparation by high-pressure homogenization. Bacterial cell wall with good adjuvant effect, bacterial protein with antigenic specificity (which includes protective capsular antigen F1) and CpG fragments which are rich in bacterial DNA, with stronger immunological competence, are concentrated in the split products; the expression vector including plague bacilli V antigens or F1 antigens which are constructed by adopting the gene recombination technology is transformed into escherichia coli, and after the inducible expression is performed, recombination plague bacilli V antigens or F1 antigens are obtained through purification. The vaccine is applicable to the general population for immunizing and preventing the plague.
Owner:NAT INST FOR THE CONTROL OF PHARMA & BIOLOGICAL PROD
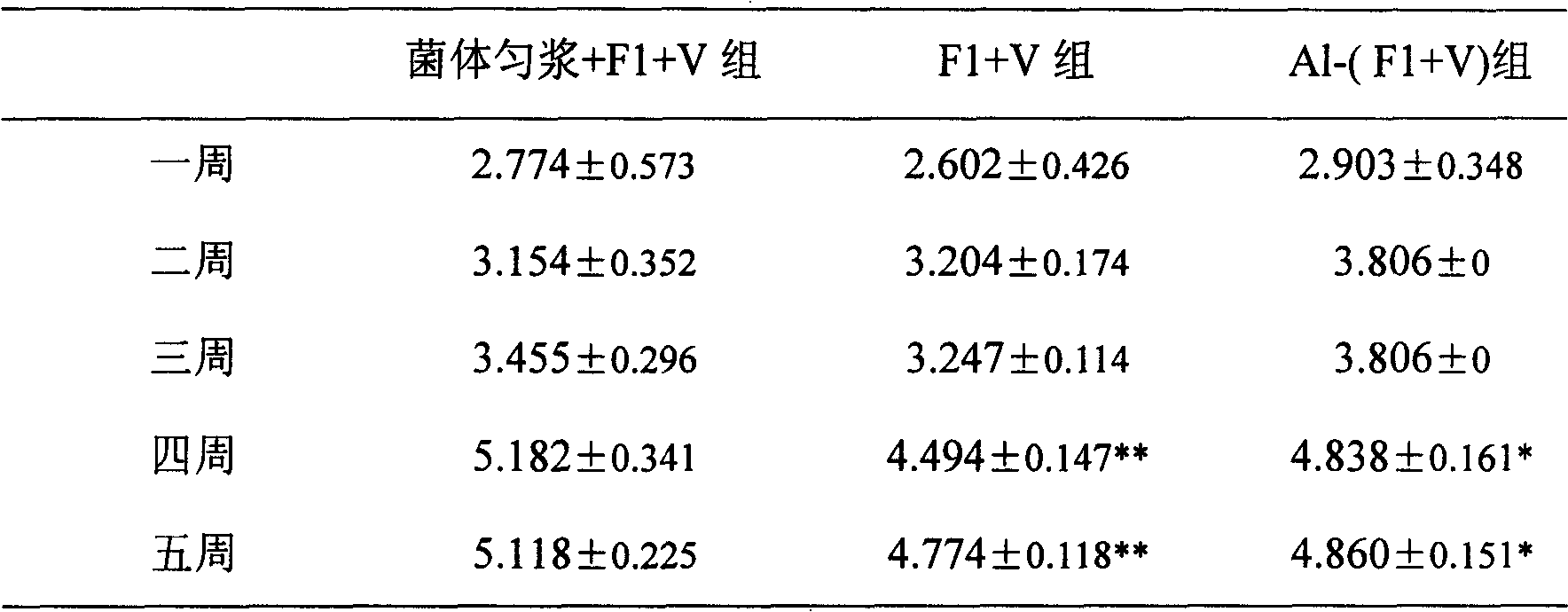
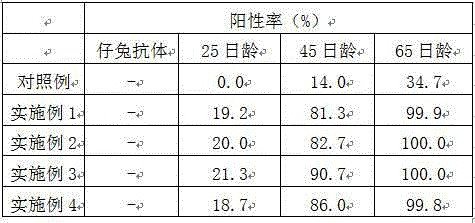
Method for improving immunity of rabbits by combining bursin and vaccine
InactiveCN105056224AImprove immunityIncrease weightViral antigen ingredientsTripeptide ingredientsPlague vaccineImmunopotency
The invention provides a method for improving the immunity of rabbits by combining bursin and a vaccine. The method for improving the immunity of rabbits comprises vaccine injection and feeding, wherein the vaccine used during vaccine injection is a mixed vaccine composed of bursin and a rabbit plague vaccine, the bursin accounts for 0.1-0.8% of the rabbit plague vaccine in mass percentage, the vaccine injection comprises the first-time injection and the second-time injection, the first-time injection is carried out when the baby rabbit is 20 days old, the use amount of bursin for each baby rabbit is 0.003-0.01ml, the second-time injection is carried out when the baby rabbit is 40 days old, and the use amount of bursin for each baby rabbit is 0.006-0.02ml. The fodder used during feeding comprises wax gourd seed powder, lily root flour, photosynthetic bacteria preparation, bacillus thermoamylovorans preparation and basic forage. With the adoption of the method, the immunity of rabbits is obviously improved, the weight of the rabbits is obviously increased, and the feed conversion ratio is reduced.
Owner:山东仙普爱瑞科技股份有限公司
Method for producing rabbit plague vaccine with cell line and its product
ActiveCN102952784BGuaranteed to be pureEnsure safetyViral antigen ingredientsMicroorganism based processesPlague vaccineCulture cell
The invention provides a method for producing RHD (rabbit haemorrhagic disease) virus and vaccine by use of common easy-to-culture cell lines such as RK13, ST, VeroE6, BHK-21, PK-15, IBRS-2 and the like. The method comprises the following steps of: inoculating the RHD virus and adsorbing; adding an incubation agent and performing cell incubation; culturing cells with a cell maintenance medium to obtain RHD virus seeds for production; inoculating the RHD virus seeds and adsorbing; adding an incubation agent and performing cell incubation; re-adding a cell maintenance medium and culturing cells to obtain the RHD virus for vaccine preparation; adding an inactivator for inactivation; and then adding a veterinary-pharmaceutically acceptable vaccine adjuvant to obtain a finished product of the RHD vaccine. According to the method, the cell line for virus culture is a mature commercial cell line, the production technology is simple and stable, the operation is easy, the virus content is high, the inter-batch difference is little, the quality is easy to control, and the output and quality of the vaccine can be obviously improved. Through the invention, the produced RHD vaccine has high safety and high immunity, and realizes a complete immune protection effect against the RHD virulent attack.
Owner:PULIKE BIOLOGICAL ENG INC
Test paper bar for testing colloidal gold of F1 antibody of plague bacterium
InactiveCN1963513APlay a role in auxiliary detectionEasy to detectMaterial analysisAntigenPlague vaccine
This invention provides one rapid test bar for plague vaccine F1 antigen, which covers plague vaccine F1 antibody and IgG on NC film and combines glue gold label plague vaccine F1 single clone antibody and applies film analysis double antibody clamper and tests label plague vaccine F1 antigen. This invention is suitable for large scale flow virus research for biological need.
Owner:BEIJING ZHUANGDI HAOHE BIOMEDICINE SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com