Compound plague vaccine and preparation method thereof
A plague vaccine and vaccine technology, applied in the field of plague vaccines, can solve problems such as short protection period, high side reaction rate, and difficulty in vaccine transportation, and achieve the effects of reducing side effects, improving safety, and improving immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: the preparation of the plague vaccine that the bacterial cell lysate of attenuated plague EV strain and recombinant V antigen and F1 antigen form
[0041] 1. Preparation of lysate of plague attenuated EV strain
[0042] 1.1. Bacterial culture: Dissolve the attenuated plague EV strain (from Lanzhou Institute of Biological Products, strain number 52006) preserved at low temperature, inoculate it on Hou's agar medium, and cultivate it at 28°C for 48 hours to form the first generation. The first generation was replanted on Hou's agar medium, and cultured at 28°C for 48 hours was the second generation, and the second generation was replanted on Hou's agar medium, and cultured at 28°C for 72 hours was the third generation.
[0043] 1.2. Bacteria collection: wash down the thalli on the surface of Thicket's agar medium with a freeze-dried protection solution (prepared according to the preferred method mentioned above).
[0044] 1.3. Preparation of the homogenate ...
Embodiment 2
[0052] Embodiment 2 safety test
[0053] In order to verify the safety of the above bacterial cell homogenate+V+F1 vaccine, the experiment was divided into four groups, each with 10 male BALB / c mice, respectively:
[0054] 1) Negative control group: normal saline 0.1ml / body
[0055] 2) Cell homogenate group: cell homogenate 56.4μg / 0.1ml / piece
[0056]3) Thale homogenate + V + F1 vaccine group: bacterial homogenate + F1, V each 10μg / 0.1ml / bird
[0057] 4) Live attenuated plague EV vaccine: 1×10 7 / 0.1ml / piece
[0058] Subcutaneous immunization was adopted on the back and observed for 7 days. Inoculate 56.4 μg bacterial cell homogenate group (equivalent to 2×10 8 Plague attenuated EV strain), thalline homogenate+V+F1 vaccine group and negative control group, no lumps were touched at the injection site, and the animals had no reverse hair, diarrhea, or other poisoning phenomena. while inoculating 1×10 7 Three of the animals that received live attenuated plague EV vaccine d...
Embodiment 3
[0060] Example 3 Immunogenicity Detection
[0061] In order to study the immunogenicity of the vaccine of the present application, the experiment was divided into four groups, with 10 female Balb / c mice in each group, respectively:
[0062] 1) Negative control group: normal saline 0.1ml / body
[0063] 2) Cell homogenate+V+F1 vaccine group: cell homogenate 30μg+F1, V each 10μg / 0.1ml / body
[0064] 3) F1+V group (combination of F1 and V antigens): 10 μg / 0.1ml of F1 and V each
[0065] 4) Al-(F1+V) group: Al(OH) 3 +F1, V each 10μg / 0.1ml / piece
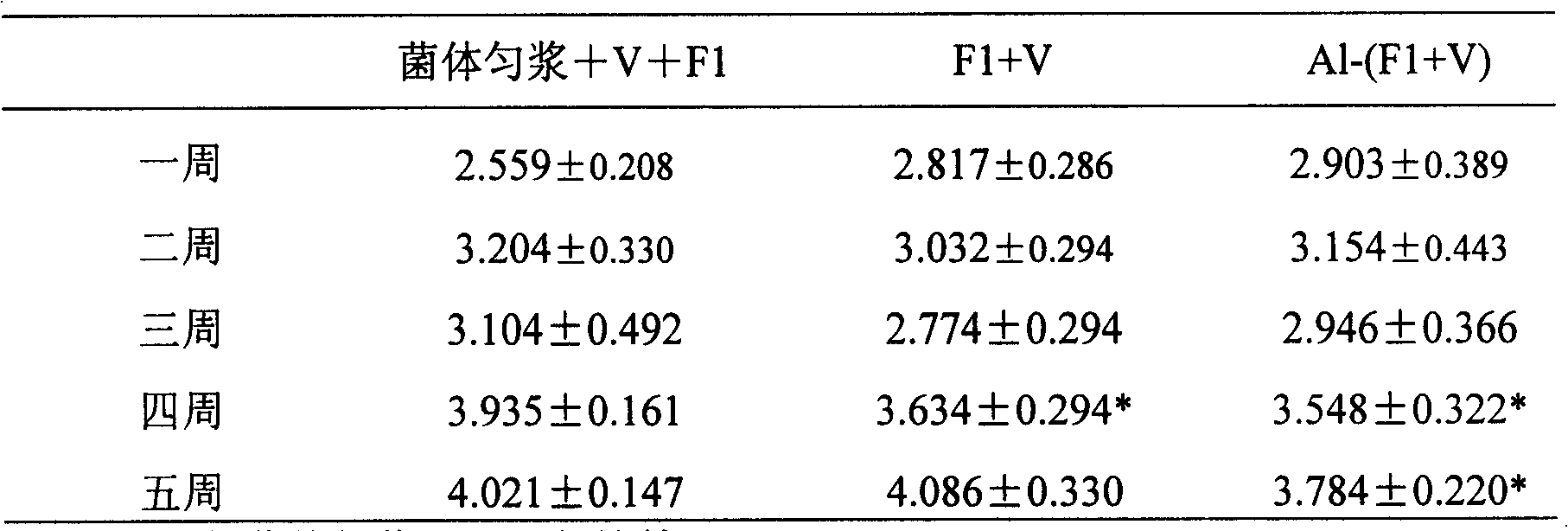
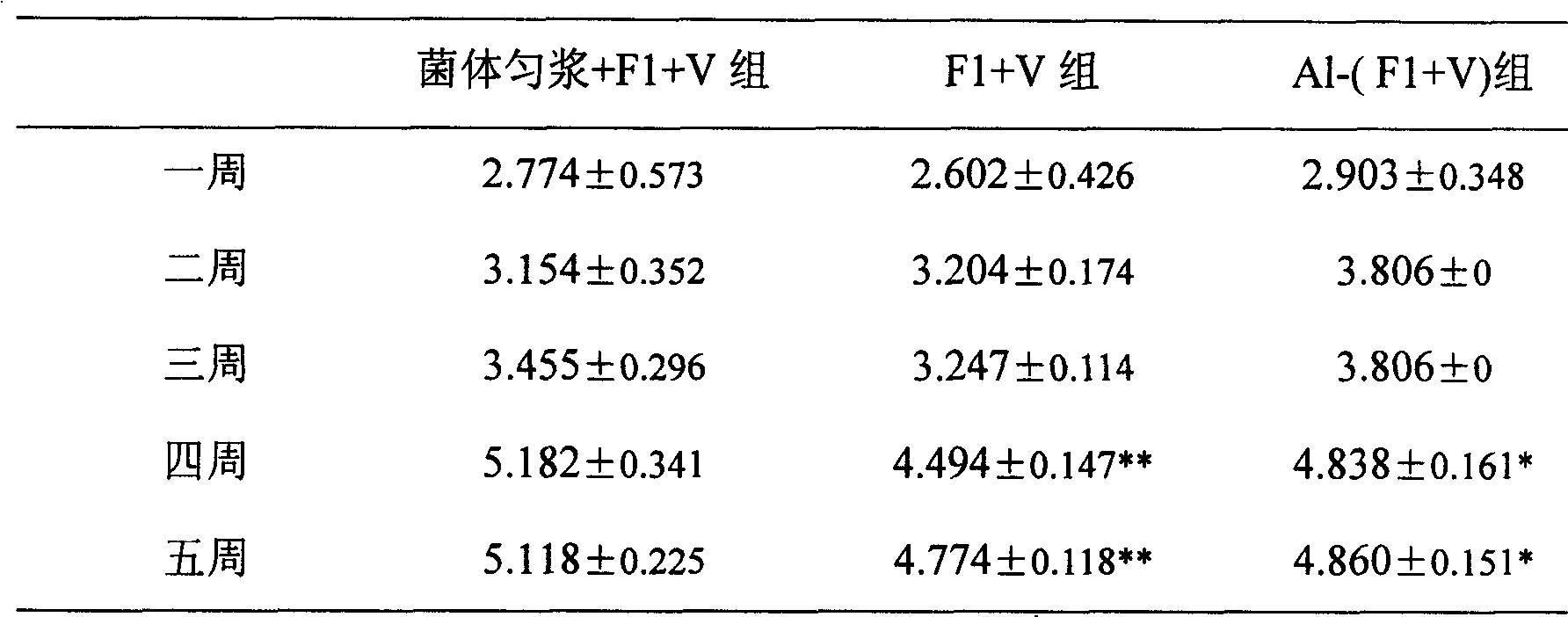
[0066] Mice were immunized subcutaneously on the back on day 0 and day 21, respectively. Blood was collected within 1, 2, 3, 4, and 5 weeks after immunization, and serum antibody titers were measured by ELISA. The results of F1 and V antibody titers are shown in Table 1 and Table 2, respectively.
[0067] Table 1 F1 antibody titer (log10) (X±SD)
[0068]
[0069] Note: * Indicates that compared with the cell homogenate+V+F1 group,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com