High-efficiency streptococcus pneumonia chimeric lyase and its mutant and application thereof
A Streptococcus pneumoniae, lysing enzyme technology, applied in the field of genetic engineering, can solve the problems that the physical and chemical properties cannot meet the requirements of Streptococcus pneumoniae infection, the number is small, and achieve the prevention and treatment of Streptococcus pneumoniae infection, good stability, and efficient killing Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Synthesis of the cell wall binding domain GPB of ACQ35_gp20
[0043] The ACQ35-gp20 gene of Streptococcus phage SpSL1 was synthesized in Nanjing GenScript Biotechnology Co., Ltd., and the synthetic sequence was loaded into pUC57 plasmid. Using the ACQ35-gp20 gene as a template and gp20-F / gp20-R as primers, the cell wall binding domain GPB of ACQ35-gp20 was amplified, its amino acid sequence is shown in SEQ ID NO.3, and its nucleotide sequence is shown in SEQ ID NO. 4. The primers and restriction site information used in the clone construction are as follows:
[0044] SEQ ID NO.11 gp20-F:5-tataggatccgttgatccgtatccgtatctg-3BamHI
[0045] SEQ ID NO.12 gp20-R:5-tatactcgagtttggtggtaatcagaccgtcc-3XhoI
[0046] The reaction system for PCR amplification of gene fragments is:
[0047]
[0048] The PCR amplification procedure is as follows:
[0049] 1) 94°C, 5min; 2) 94°C, 30sec, 62°C, 45sec, 72°C, 45sec, 30 cycles; 3) 72°C, 10min.
Embodiment 2
[0050] Example 2: Construction and expression of EGFP-GPB and EGFP
[0051] 1. The ACQ35-gp20 gene of Streptococcus phage SpSL1 was synthesized in Nanjing GenScript Biotechnology Co., Ltd., and the synthetic sequence was loaded into the pUC57 plasmid. Using the pEGFP plasmid as a template and EGFP-F / EGFP-R as primers, the sequence of enhanced green fluorescent protein EGFP was amplified. The primers and restriction site information used in the construction of the clone are as follows:
[0052] SEQ ID NO.13 EGFP-F:5-tataccatggtgagcaagggcgaggagc-3NcoI
[0053] SEQ ID NO.14 EGFP-R:5-tataggatcccttgtacagctcgtccatgc-3BamHI
[0054] The reaction system for PCR amplification of gene fragments is:
[0055]
[0056]
[0057] The PCR amplification procedure is as follows:
[0058] 1) 94°C, 5min; 2) 94°C, 30sec, 62°C, 45sec, 72°C, 45sec, 30 cycles; 3) 72°C, 10min.
[0059] 2. The above fragment and the cell wall binding domain GPB of ACQ35-gp20 in Example 1 were digested with co...
Embodiment 3
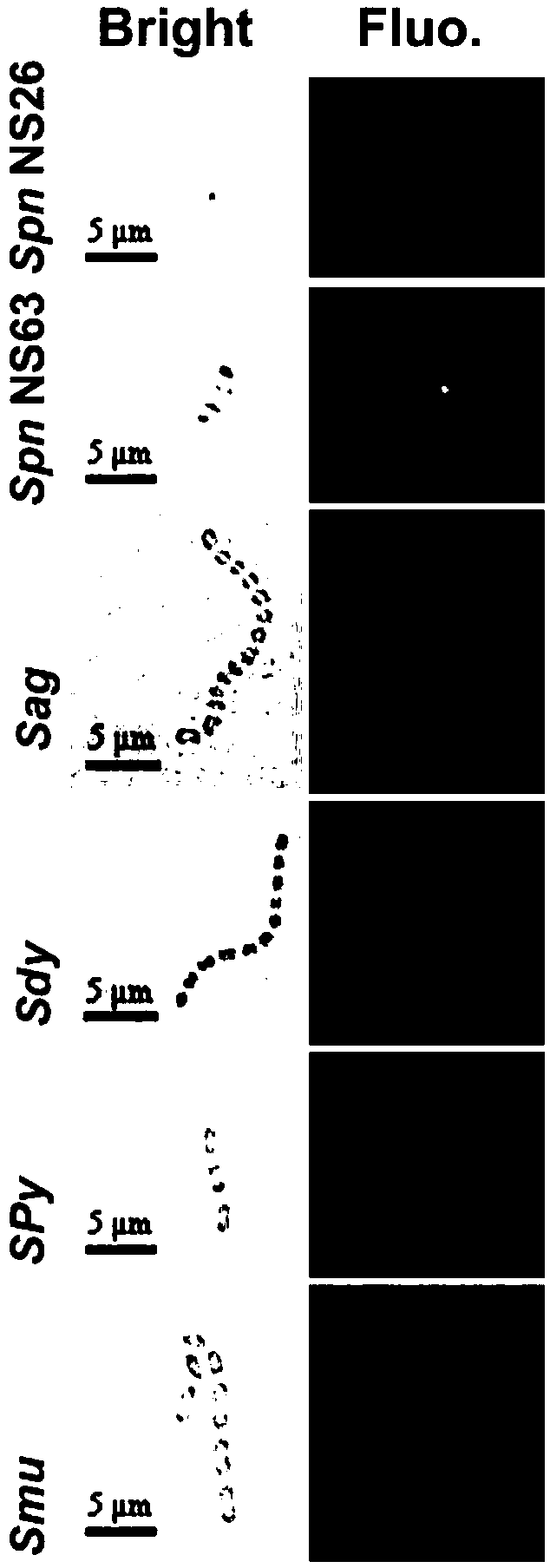
[0061] Example 3: Validation of EGFP-GPB binding to Streptococcus pneumoniae
[0062] Overnight cultures of streptococci including Streptococcus pneumoniae NS26 (Spn NS26), Streptococcus pneumoniae NS63 (SpnNS63), Streptococcus agalactiae (Sag), Streptococcus dysgalactiae (Sdy), Streptococcus pyogenes (Spy), and Streptococcus mutans For cocci (Smu), wash once with PBS and resuspend to OD600=0.5-0.8, then take 0.01 ml of the treated bacteria solution and incubate with 20 μM EGFP-GPB protein at 37 degrees for 1 hour. Wash 3 times with PBS to remove unbound protein, and observe the cells under a fluorescent microscope. At the same time, under the same experimental conditions, EGFP protein was used as a control protein for binding experiments. The result obtained is as figure 2 shown. The results showed that EGFP-GPB could specifically bind to Streptococcus pneumoniae strains.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com