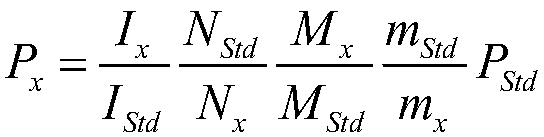Method for accurately measuring purity of volatile substances by adopting quantitative HNMR
A technology of proton nuclear magnetic resonance spectroscopy and volatility, which is applied in the field of detection and analysis, and can solve problems that affect the accuracy of measurement results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The purity of methyl tert-butyl ether (MTBE) was accurately measured by quantitative proton nuclear magnetic spectroscopy.
[0038] Target sample: methyl tert-butyl ether, a colorless, low-viscosity liquid, slightly soluble in water, miscible with many organic solvents, with a boiling point of 55°C, molecular formula C 5 h 12 O, the molar mass is 88.15. The sample was purchased from Sigma Company, and the gas chromatography (GC) purity was 99.94%.
[0039] At first adopt routine technology to measure: internal standard is the commonly used quantitative NMR solid internal standard acesulfame potassium (GBW (E) 100065, purity 99.6%, expanded uncertainty 0.6%, k=2), molecular formula C 4 h 4 KNO 4 S, the molar mass is 201.24.
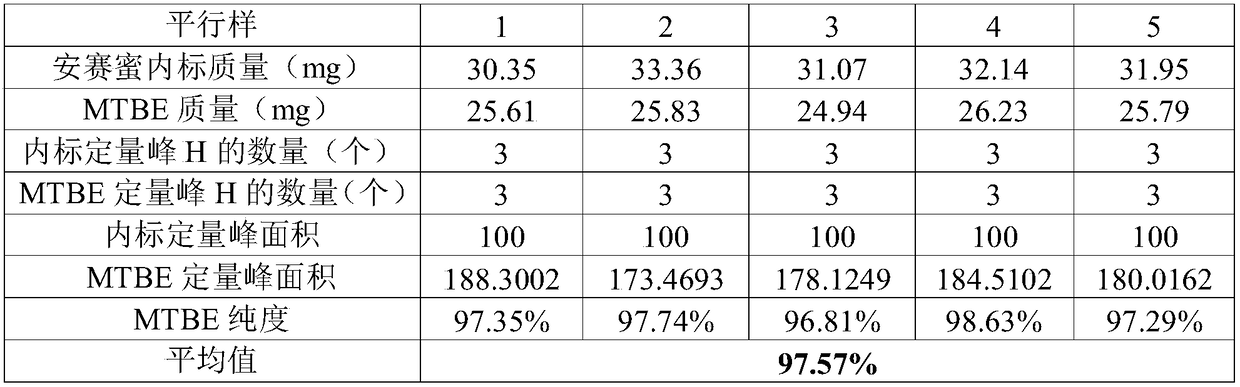
[0040] Weigh about 32mg of acesulfame potassium solid internal standard into a 1.5mL solution bottle, weigh the mass of the internal standard, accurate to 0.01mg; use a pipette to pipette about 25mg of methyl tert-butyl ether, add the solution ...
Embodiment 2
[0050] The purity of tert-amyl methyl ether (TAME) was accurately measured by quantitative proton nuclear magnetic spectroscopy.
[0051] Target sample: methyl tert-amyl ether, boiling point 85°C, molecular formula C 6 h 14 O, the molar mass is 102.18. The sample was purchased from Sigma Company, and the gas chromatography (GC) purity was 98.60%.
[0052] Adopt the method of the present invention, the internal standard selects national primary standard substance ethyl acetate (GBW06114, purity 99.7%, expanded uncertainty 0.4%, k=2), boiling point 77.5 ℃, molecular formula C 4 h 8 o 2 , with a molar mass of 88.11.
[0053] Transfer 0.6mL of deuterated chloroform to a 1.5mL sealed sample bottle with a 1000 μL airtight needle, transfer about 19 mg of internal standard ethyl acetate into the sample bottle with a 50 μL airtight needle, and use a high-precision balance (d = 0.01 mg) weigh the mass of ethyl acetate; then use another 50 μL airtight needle to transfer about 15 mg...
Embodiment 3
[0058] The purity of isopropanol (IPA) was accurately measured by quantitative proton nuclear magnetic spectroscopy.
[0059] Target sample: Isopropanol, a colorless transparent liquid with an odor like a mixture of ethanol and acetone. Soluble in water, also soluble in alcohol, ether, benzene, chloroform and most other organic solvents. Boiling point is 82°C, molecular formula C 3 h 8 O, the molar mass is 60.1. The sample was purchased from Sigma Company, and the gas chromatography (GC) purity was 99.98%.
[0060] Adopt the method of the present invention, the internal standard selects national primary standard substance methanol (GBW06111, purity 99.7%, expanded uncertainty 0.3%, k=2), boiling point 65.4 ℃, molecular formula CH 4 O, the molar mass is 32.04.
[0061] Transfer 0.6mL deuterated dimethyl sulfoxide to a 1.5mL sealed sample vial with a 1000 μL airtight needle, transfer about 29 mg of internal standard methanol into the sample vial with a 50 μL airtight needle...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Boiling point | aaaaa | aaaaa |
| Boiling point | aaaaa | aaaaa |
| Boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com