Glycosyltransferase, mutants and application thereof
A technology of glycosyltransferase, glycosyl, applied in the field of biotechnology and plant biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 10
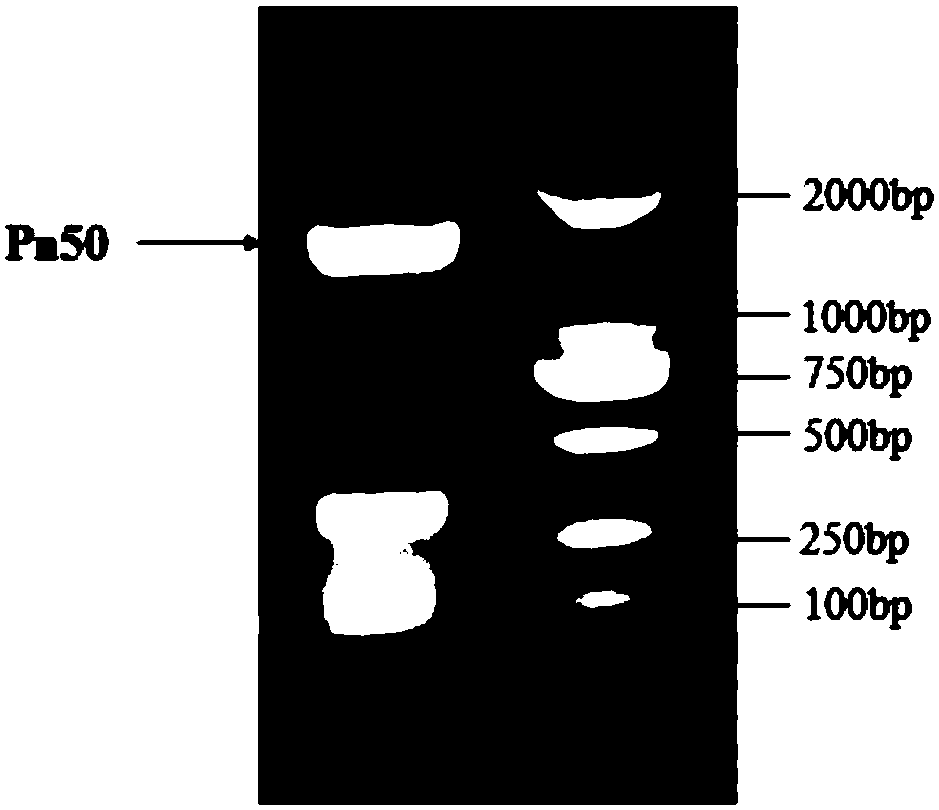
[0213] Example 1. Cloning of the notoginseng glycosyltransferase gene Pn50
[0214] Two primers such as Pn50 cloning primer F, SEQ ID NO: 1 (ATGGAGAGAGAAATGTTGAGCA) and Pn50 cloning primer R, SEQ ID NO: 2 (TCAGGAGGAAACAAGCTTTGAA) were synthesized. Using the cDNA obtained by reverse transcription of the RNA extracted from Panax notoginseng as a template, PCR was performed using the above primers. The DNA polymerase was selected from the high-fidelity KOD DNA polymerase of Treasure Bioengineering Co., Ltd. The PCR amplification program was as follows: 94°C for 2min; 94°C for 15s, 58°C for 30s, 68°C for 2min, a total of 35 cycles; 68°C for 10min to 10°C. The PCR products were detected by agarose gel electrophoresis, and the results were shown in figure 1 .
[0215] Under UV irradiation, the target DNA band is excised. Then, Axygen Gel Extraction Kit (AEYGEN Company) was used to recover DNA from the agarose gel, which was the DNA fragment of the amplified glycosyltransferase g...
Embodiment 2 10
[0221] Example 2. Expression of the notoginseng glycosyltransferase gene Pn50 in Escherichia coli
[0222] Two primers such as the sequence Pn50 expression primer F SEQ ID NO: 5 (GGATCCATGGAGAGAGAAATGTTGAGCA) and Pn50 expression primer R SEQ ID NO: 6 (CTCGAGTCAGGAGGAAACAAGCTTTGAA) were synthesized. Two restriction sites, BamH I and Xho I, were respectively added to the synthesized primers F / R, and PCR was carried out using cDNA extracted from plants as a template. The DNA polymerase was selected from the high-fidelity KOD DNA polymerase of Treasure Bioengineering Co., Ltd. The PCR amplification program was as follows: 94°C for 2min; 94°C for 15s, 58°C for 30s, 68°C for 2min, a total of 35 cycles; 68°C for 10min to 10°C. PCR products were detected by agarose gel electrophoresis. Under UV irradiation, the target DNA band is excised. Then, Axygen Gel Extraction Kit (AEYGEN Company) was used to recover DNA from the agarose gel, which was the DNA fragment of the amplified glycos...
Embodiment 3 10
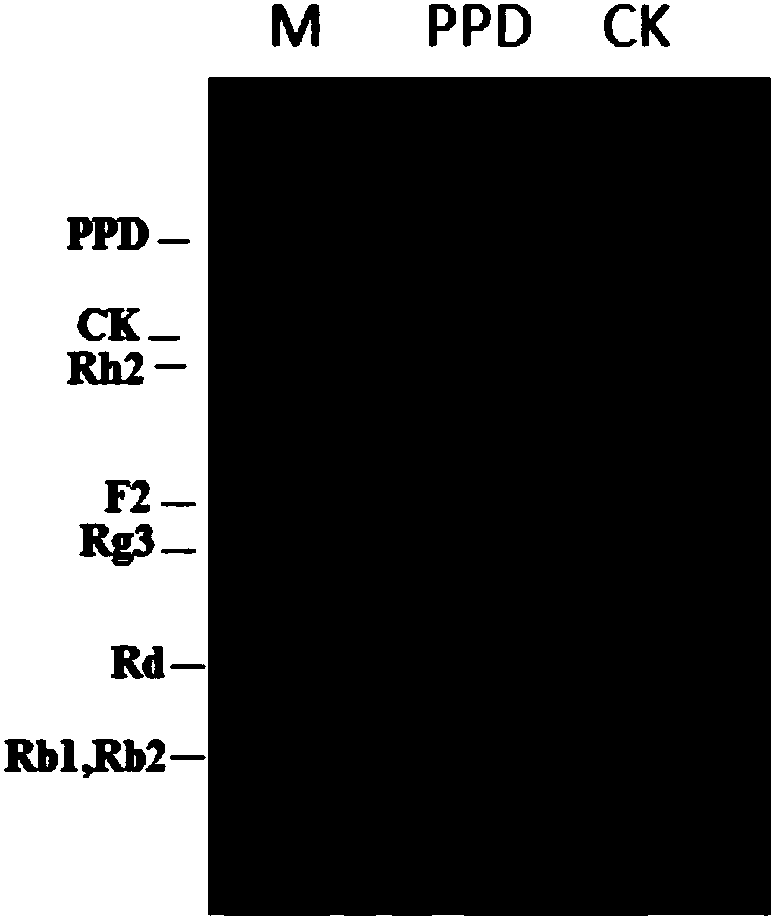
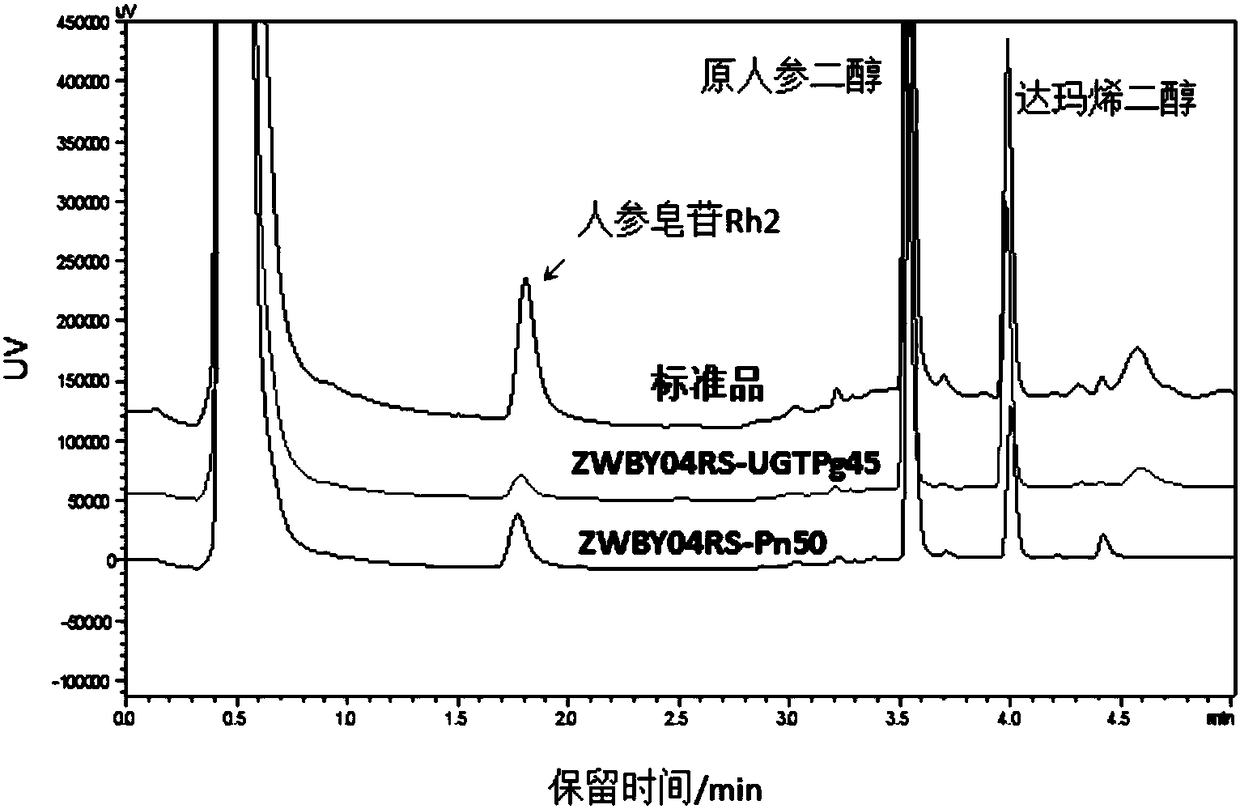
[0223] Example 3. Notoginseng glycosyltransferase gene Pn50 catalyzes different substrate reactions and detection of its products
[0224] Configure the glycosyltransferase Pn50 catalytic reaction system (100 μL) as follows:
[0225]
[0226] The reaction was carried out in a water bath at 37°C for 2h. After the reaction was completed, an equal volume of n-butanol was added for extraction, and the n-butanol phase was taken, concentrated in vacuo, and the reaction product was dissolved in 10 μL of methanol, and the results were detected by TLC or HPLC. From figure 2 As can be seen from the results in the present invention, the glycosyltransferase Pn50 used in the present invention can catalyze protopanaxadiol PPD to form a new product (see formula A for the reaction), and its migration position on the TLC plate is consistent with that of Rh2. Prove that this new product is ginsenoside Rh2. In addition, Pn50 can also catalyze compound K, and the generated product is specu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Theoretical molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com