Preparation method of self-supporting electrode material, self-supporting electrode material and electrolysis apparatus
A self-supporting electrode and electrode material technology, applied in the direction of electrodes, electrolysis process, electrolysis components, etc., can solve the problems of reducing the contact area between active materials and electrolyte, product conversion rate and selectivity, uneven catalyst coating, etc., to achieve The effect of accelerating electron collection and transfer rate, increasing electrode reaction rate, and improving conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
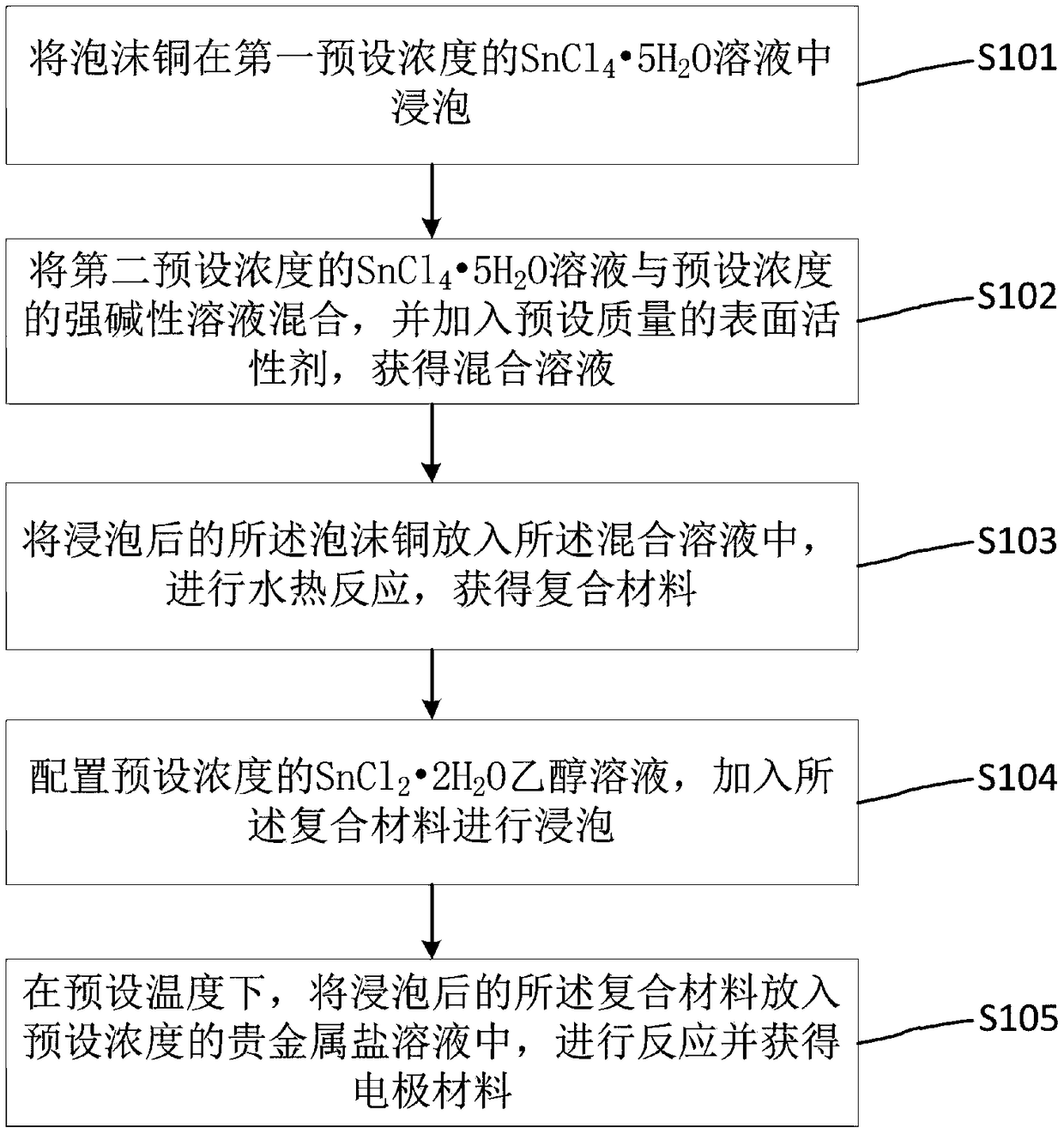
[0029] refer to figure 1 As shown, the embodiment of the present invention provides a method for preparing a self-supporting electrode material, including:
[0030] Step 1 S101: adding foamed copper to SnCl with a first preset concentration 4 ·5H 2 Soak in O solution;
[0031] Step 2 S102: adding the second preset concentration of SnCl 4 ·5H 2 O solution is mixed with a strong alkaline solution with a preset concentration, and a surfactant with a preset quality is added to obtain a mixed solution;
[0032] Step 3 S103: putting the soaked copper foam into the mixed solution, and performing a hydrothermal reaction to obtain a composite material;
[0033] Step 4 S104: Configuring SnCl with a preset concentration 2 2H 2 0 ethanol solution, add the composite material and soak;
[0034] Step 5 S105: at a preset temperature, put the soaked composite material into a noble metal salt solution with a preset concentration to react and obtain an electrode material.
[0035] Speci...
Embodiment 1
[0059] 1. Cut 2x3cm copper foam and ultrasonically clean it in 40ml of acetone for 10 minutes to remove the oil stains on the surface; then put it into 30ml of dilute hydrochloric acid with a mass fraction of 1% and ultrasonically clean it for 5 minutes to remove the oxides on the surface, and then use it separately Wash with deionized water and absolute ethanol three times, and finally dry in a vacuum oven at 60°C for 1 h.
[0060] 2. Take the treated foam copper in step 1 and soak it in 0mol / L SnCl 4 ·5H 2 O solution for 1 hour. Afterwards, they were washed three times with deionized water and absolute ethanol, and finally dried in a vacuum oven at 60 °C for 0 h.
[0061] 3. Take 0.8g of SnCl respectively 4 ·5H 2 O and 40ml of deionized water to make solution A, take 0.8g of NaOH and 40ml of deionized water to make solution B, under the condition of magnetic stirring, slowly add solution A to solution B, continue stirring for 10min and then add 1g CTAB, continue stirrin...
Embodiment 2
[0067] 1. Cut 2x3cm copper foam and ultrasonically clean it in 40ml of acetone for 15 minutes to remove the oil stains on the surface; then put it into 30ml of dilute hydrochloric acid with a mass fraction of 2.5% and ultrasonically clean it for 10 minutes to remove the oxide on the surface, and then use it separately Wash with deionized water and absolute ethanol three times, and finally dry in a vacuum oven at 70°C for 3 hours.
[0068] 2. Take the treated foam copper in step 1 and soak it in 0.05mol / L SnCl 4 ·5H 2 O solution for 2.5 hours. Afterwards, they were washed three times with deionized water and absolute ethanol, and finally dried in a vacuum oven at 70°C for 1 h.
[0069] 3. Take 12g of SnCl respectively 4 ·5H 2 O and 40ml of deionized water to make solution A, take 4g of NaOH and 40ml of deionized water to make solution B, under the condition of magnetic stirring, slowly add solution A to solution B, continue stirring for 10min and then add 1.48g CTAB, conti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com