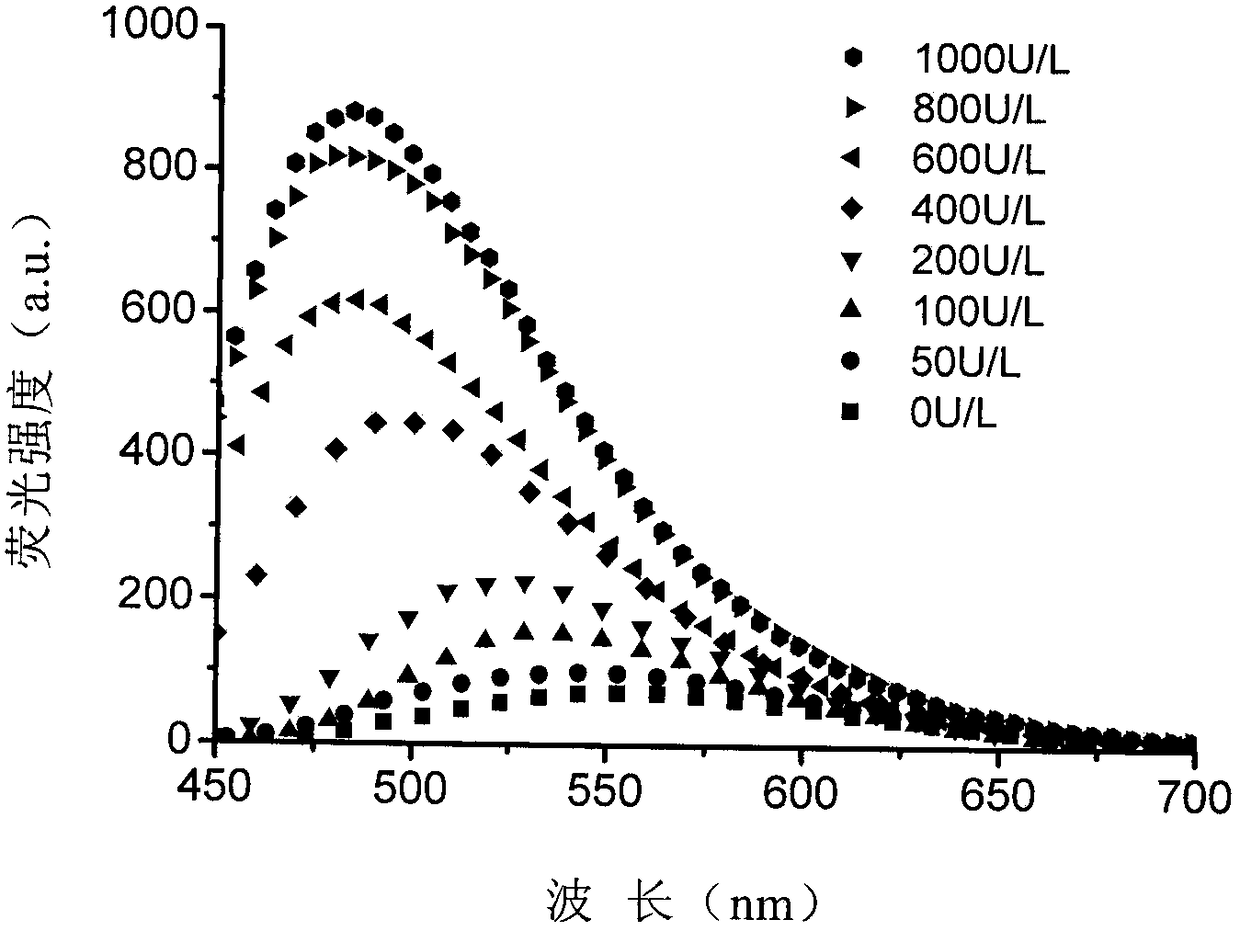
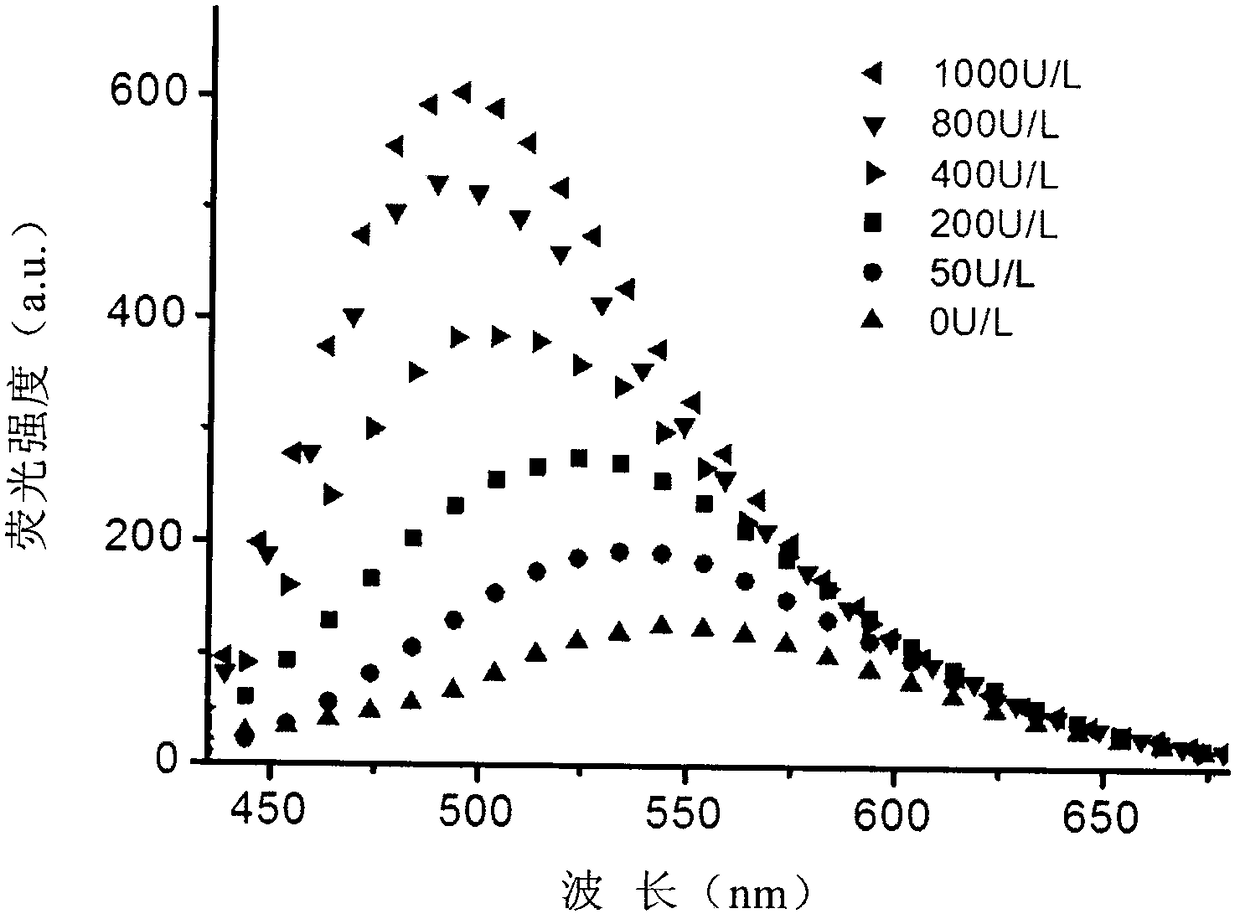
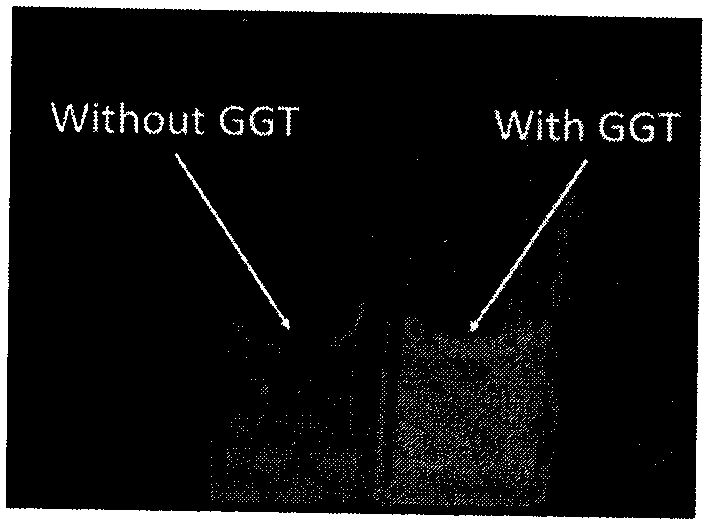
Fluorescent probe for detecting high-concentration gamma-Glutamyl Transpeptidase and preparation method thereof
A technology of glutamyl transpeptidase and fluorescent probe, which is applied in the field of analytical chemistry, and achieves the effects of low preparation cost, easy promotion, and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Synthesis of TAB-3-GSH
[0056]
[0057] (1) Preparation of Compound 2: Under the protection of nitrogen, 10g (32mmol) of Compound 2 was dissolved in 60mL of dry ether, cooled to -78°C, and 20mL of 1.6M n-butyllithium n-hexane was added dropwise. Hexane solution. The reaction was warmed to room temperature and stirred for 20 min. Then, it was cooled to -78°C again, and 1.25 mL (10 mmol) of boron trifluoride ether was added. The reaction continued at room temperature and stirred overnight. The solvent was spin-dried, and further purified by silica gel column chromatography to obtain 4.0 g of white solid with a yield of 71%.
[0058] (2) Preparation of compound 3: Put 595 mg (3.2 mmol) of N-butyl ester piperazine, 560 mg (1 mmol) of compound 2, 864 mg (9 mmol) of sodium tert-butyl alkoxide, and 27 mg (0.12 mmol) of palladium acetate into three mouths In the bottle, pump three times on a double-row tube, under the protection of nitrogen, add 50 mL of toluene, and then add 2...
Embodiment 2
[0063] Synthesis of TAB-3-Cys-Gly
[0064]
[0065] (1) Preparation of Compound 2: Under the protection of nitrogen, 10g (32mmol) of Compound 2 was dissolved in 60mL of dry ether, cooled to -78°C, and 20mL of 1.6M n-butyllithium n-hexane was added dropwise. Hexane solution. The reaction was warmed to room temperature and stirred for 20 min. Then, it was cooled to -78°C again, and 1.25 mL (10 mmol) of boron trifluoride ether was added. The reaction continued at room temperature and stirred overnight. The solvent was spin-dried, and further purified by silica gel column chromatography to obtain 4.0 g of white solid with a yield of 71%.
[0066] (2) Preparation of compound 3: Put 595 mg (3.2 mmol) of N-butyl ester piperazine, 560 mg (1 mmol) of compound 2, 864 mg (9 mmol) of sodium tert-butyl alkoxide, and 27 mg (0.12 mmol) of palladium acetate into three mouths In the bottle, pump three times on a double-row tube, under the protection of nitrogen, add 50 mL of toluene, and then a...
Embodiment 3
[0071] Synthesis of TAB-3-Cys
[0072]
[0073] (1) Preparation of Compound 2: Under the protection of nitrogen, 10g (32mmol) of Compound 2 was dissolved in 60mL of dry ether, cooled to -78°C, and 20mL of 1.6M n-butyllithium n-hexane was added dropwise. Hexane solution. The reaction was warmed to room temperature and stirred for 20 min. Then, it was cooled to -78°C again, and 1.25 mL (10 mmol) of boron trifluoride ether was added. The reaction continued at room temperature and stirred overnight. The solvent was spin-dried, and further purified by silica gel column chromatography to obtain 4.0 g of white solid with a yield of 71%.
[0074] (2) Preparation of compound 3: Put 595 mg (3.2 mmol) of N-butyl ester piperazine, 560 mg (1 mmol) of compound 2, 864 mg (9 mmol) of sodium tert-butyl alkoxide, and 27 mg (0.12 mmol) of palladium acetate into three mouths In the bottle, pump three times on a double-row tube, under the protection of nitrogen, add 50 mL of toluene, and then add 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com