Host red luminous material with charge transferring structure and its prepn and application
A red-emitting, host-based technology, applied in luminescent materials, chemical instruments and methods, can solve problems such as increasing the concentration of objects, reducing device efficiency, and incomplete matching, and achieves simple preparation processes, good thermal stability, and good solubility. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
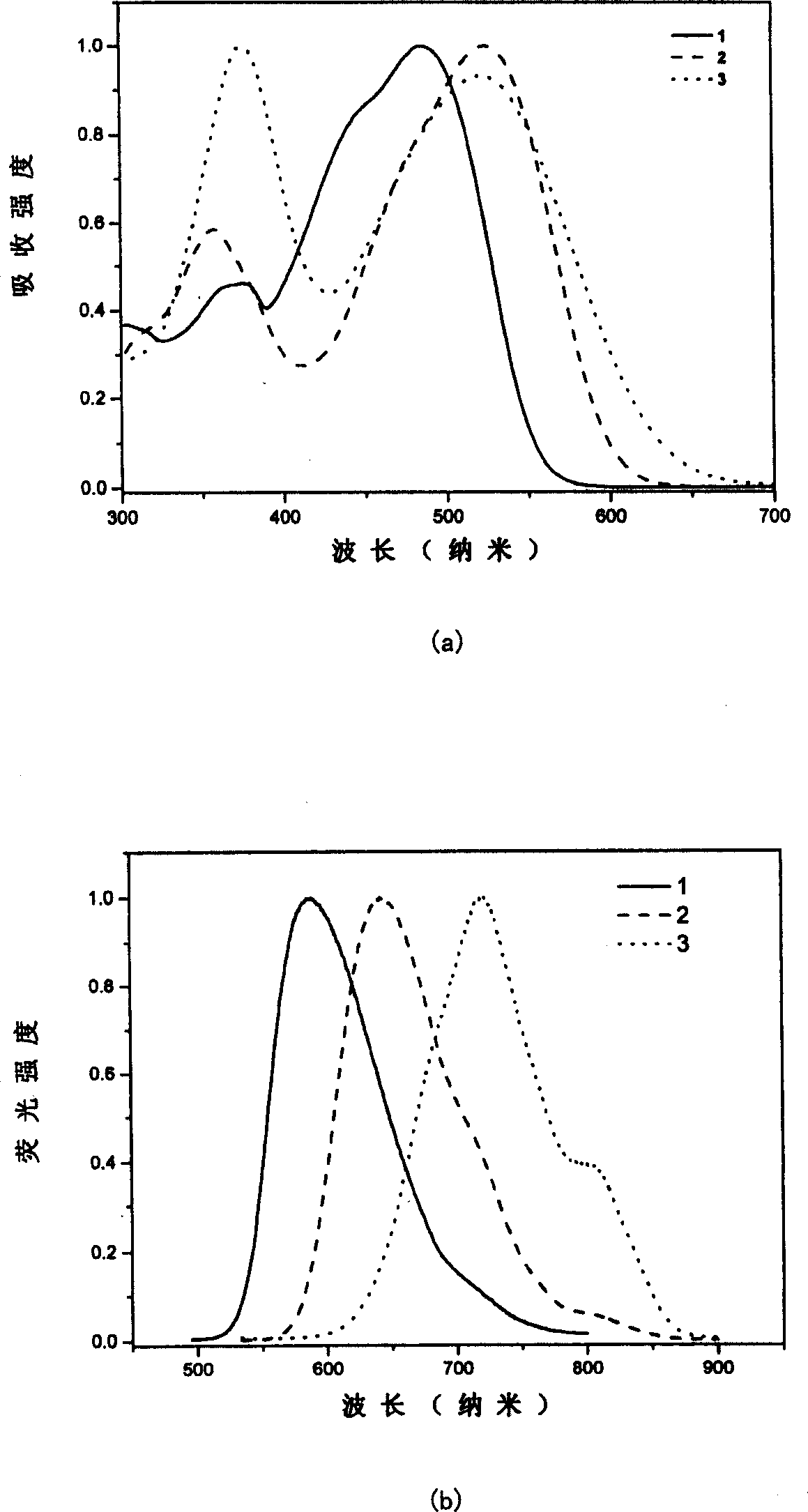
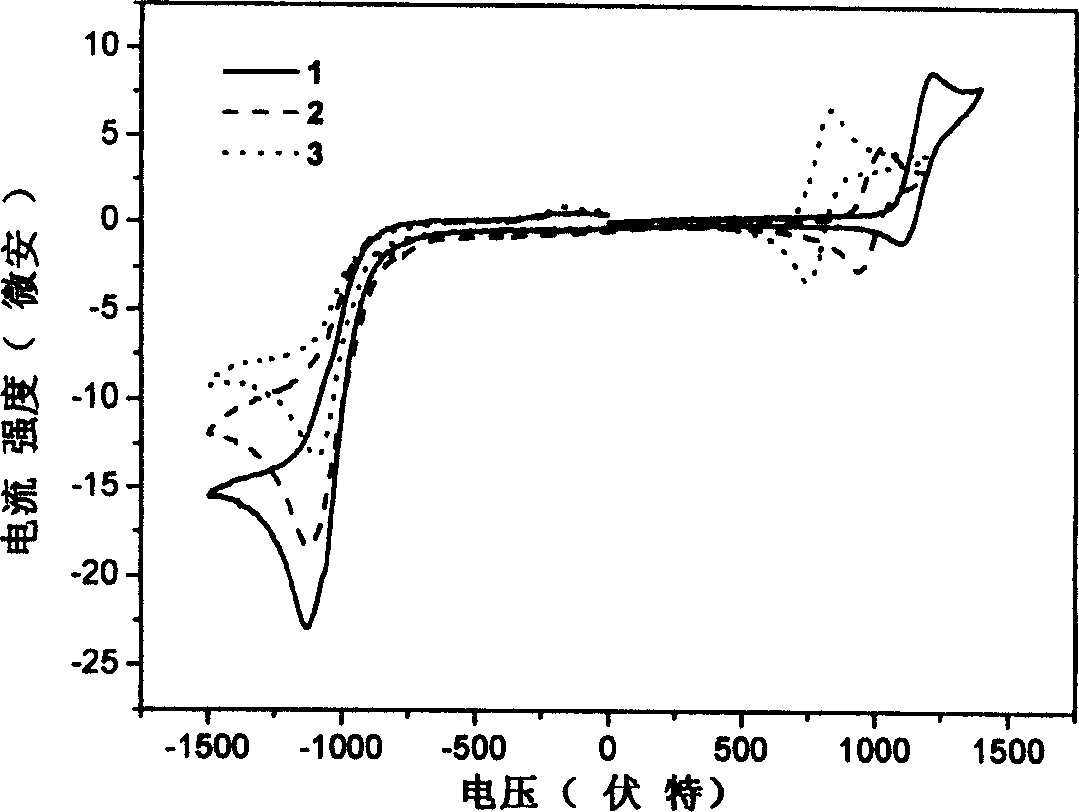
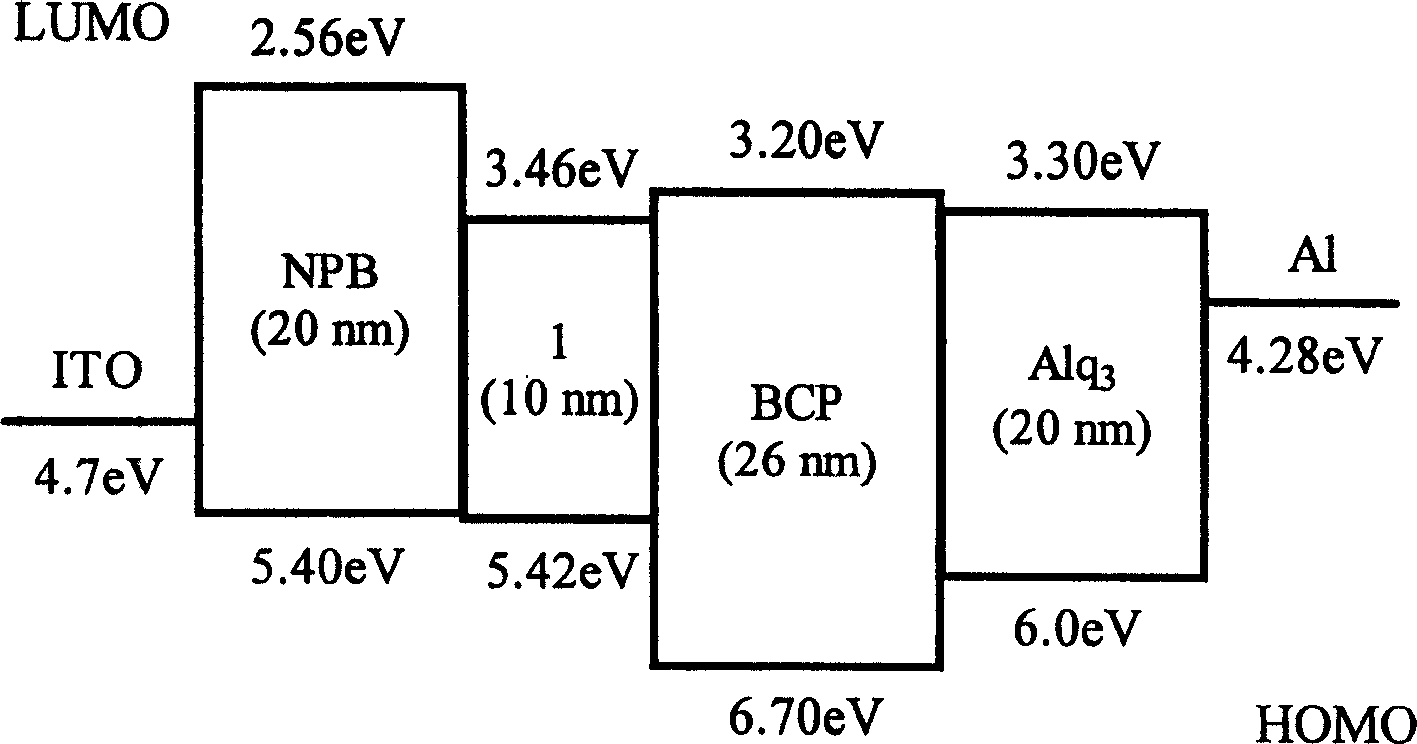
Image
Examples
Embodiment 1
[0045] 3,5-bis(2'-dicyanomethenyl-4',4'-dimethyl-cyclohexenyl)methenyl-9-propyl-carbazole (compound 1)
[0046]
[0047] In a 100ml three-necked flask, mix 3,6-dialdehyde-9-propyl-carbazole (2mmol) and 2-dicyanomethenyl-4,4,6-trimethyl-cyclohexene (8mmol ) was placed in about 20ml of acetonitrile, 3ml of piperidine was added as a catalyst, and heated to reflux at 80°C for 10 hours under a nitrogen atmosphere; after the reaction stopped, cooled to room temperature, extracted with water / dichloromethane (60ml×2); combined organic phase, dried over anhydrous magnesium sulfate. The organic solvent was removed by vacuum rotary evaporation, and the crude product was purified by silica gel column chromatography (eluent-dichloromethane / petroleum ether 2:1 v / v) to obtain a dark red solid with metallic luster (yield 82%).
[0048] Mass spectrum (MALDI-TOF-MS): m / z calculated value 601, measured value 601.6 (M + );
[0049] H NMR spectrum ( 1 H-NMR) (400MHz, CDCl 3 , ppm): δ=1.01(...
Embodiment 2
[0053] 4,4'-bis(2'-dicyanomethenyl-4',4'-dimethyl-cyclohexenyl)methenyl-triphenylamine (compound 2)
[0054]
[0055] In a 100ml three-necked flask, put 4.4'-dialdehyde-triphenylamine (2mmol) and 2-dinitrile methyl-4,4,6-trimethyl-cyclohexene (8mmol) in about 20ml of acetonitrile , add 3ml of piperidine as a catalyst, and heat to reflux at 80°C for 10 hours under a nitrogen atmosphere; after the reaction stops, cool to room temperature and extract with water / dichloromethane (60ml×2); dry. The organic solvent was removed by vacuum rotary evaporation, and the crude product was purified by silica gel column chromatography (eluent-dichloromethane / petroleum ether 2:1 v / v) to obtain a dark red solid with metallic luster (yield 72%).
[0056] Mass spectrometry (TOF-MS-EI + )m / z: calculated value 637; measured value 637;
[0057] H NMR spectrum ( 1 H-NMR) (300MHz, CDCl 3 , ppm): δ=1.08(s, 12H), 2.46(s, 4H), 2.60(s, 4H), 6.82(s, 2H), 6.92-6.99(m, 4H), 7.07-7.09(t, 4H ), 7.17(t...
Embodiment 3
[0061] 3,5-bis(2'-dicyanomethenyl-4',4'-dimethyl-cyclohexenyl)methenyl-9-propyl-phenothiazine (compound 3)
[0062]
[0063] In a 100ml three-necked flask, 3,6-dialdehyde-9-propyl-phenothiazine (2mmol) and 2-dicarbonitrile-4,4,6-trimethyl-cyclohexene ( 8mmol) was placed in about 20ml of acetonitrile, 3ml of piperidine was added as a catalyst, under nitrogen atmosphere, heated to reflux at 80°C for 10 hours; after the reaction was stopped, cooled to room temperature, extracted with water / dichloromethane (60ml×2); combined The organic phase was dried over anhydrous magnesium sulfate. The organic solvent was removed by vacuum rotary evaporation, and the crude product was purified by silica gel column chromatography (eluent-dichloromethane / petroleum ether 2:1 v / v) to obtain a purple-red solid with metallic luster (yield 78%).
[0064] Mass spectrum (MALDI-TOF-MS): m / z calculated 633; measured 633.1.
[0065] H NMR spectrum ( 1 H-NMR) (300MHz, CDCl 3 , ppm): δ=1.03(t, 3H),...
PUM
| Property | Measurement | Unit |
|---|---|---|
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com