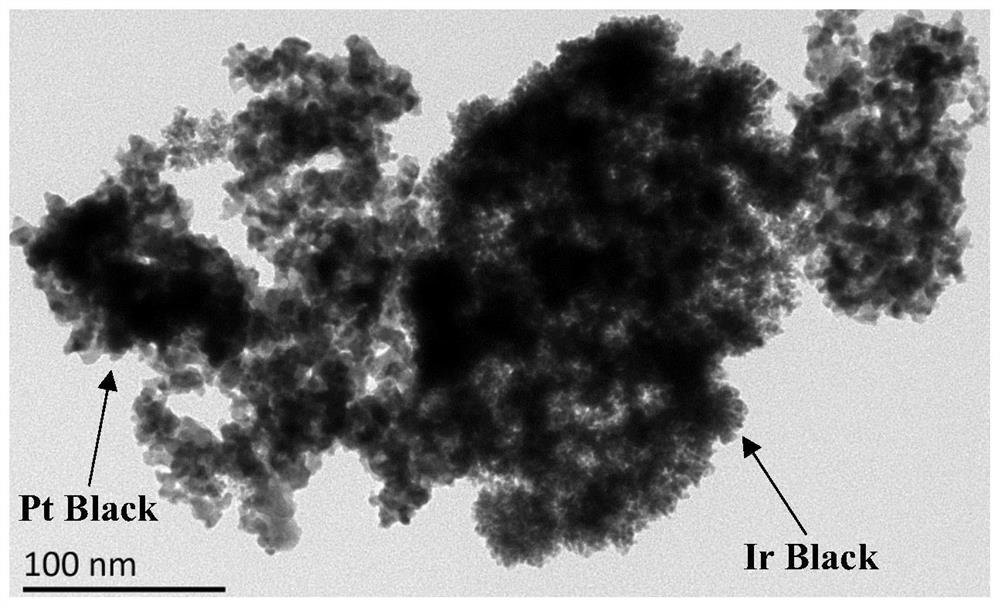
Preparation and application of a double-effect oxygen electrode catalyst with metal iridium surface-modified platinum
A double-effect oxygen electrode and catalyst technology, applied in the field of electrochemistry, can solve problems such as unfavorable catalytic reaction, unfavorable cleaning of catalysts, etc., and achieve the effects of improving oxygen reduction performance, easy control of reaction conditions, and sacrificing oxygen evolution activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Accurately weigh 19.25mg of iridium black (0.1mmol) into 100mL of deionized water, and ultrasonically disperse for 15min.
[0040] (2) Heat the above dispersion to 30°C, stir for 15 minutes and then 20ml K 2 PtCl 4(0.0001mol / L), so that the ratio of the amount of Pt to Ir is 0.02:1, and then stirred and reacted for 3h.
[0041] (3) After the reaction, it was naturally cooled to room temperature, then centrifuged, and then washed 4 times with a mixed solution of deionized water and ethanol to obtain a double-effect oxygen electrode catalyst.
Embodiment 2
[0043] (1) Accurately weigh 19.2mg of iridium black (0.1mmol) into 50mL of deionized water, and ultrasonically disperse for 15min.
[0044] (2) Heat the above dispersion to 70°C, stir for 15 minutes and then 20ml K 2 PtCl 4 (0.001mol / L), so that the ratio of the amount of Pt to Ir is 0.2:1, and then stirred and reacted for 12h.
[0045] (3) After the reaction, it was naturally cooled to room temperature, then centrifuged, and then washed 4 times with a mixed solution of deionized water and ethanol to obtain a double-effect oxygen electrode catalyst.
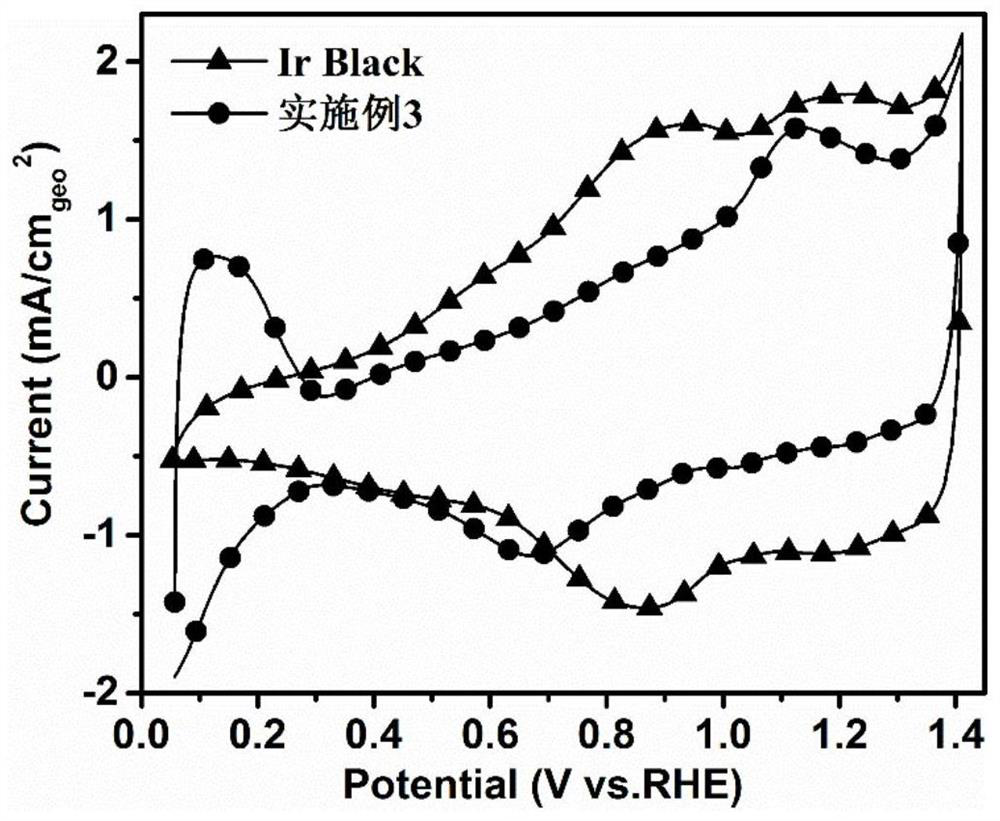
[0046] Depend on Figure 4 It can be seen that the ORR activity of the catalyst prepared in Example 2 is much higher than that of the comparative catalyst.
Embodiment 3
[0048] (1) Accurately weigh 19.2mg of iridium black (0.1mmol) into 20mL of deionized water, and ultrasonically disperse for 15min.
[0049] (2) Bring the dispersion of the above solution to 100°C, stir for 15 minutes and then add 4ml K 2 PtCl 4 (0.005mol / L), so that the ratio of the amount of substance of Pt and Ir is 0.2:1, then stirred and reacted for 12h.
[0050] (3) After the reaction, it was naturally cooled to room temperature, then centrifuged, and then washed 4 times with a mixed solution of deionized water and ethanol to obtain a double-effect oxygen electrode catalyst.
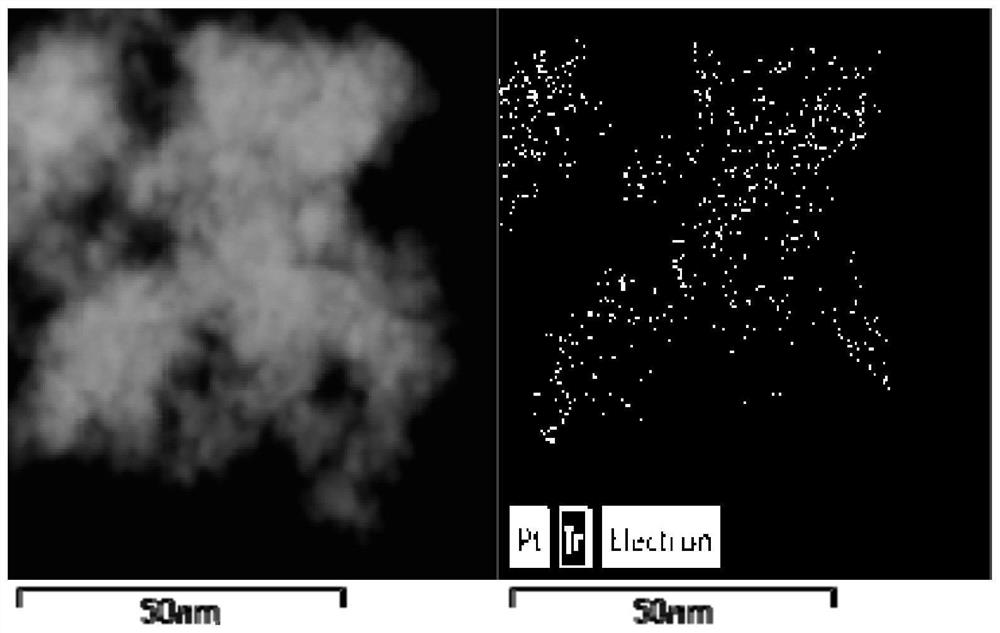
[0051] from diagram 2-1 , Figure 2-2 It can be seen that highly dispersed Pt nanoparticles were successfully modified on the surface of IrBlakck, and this structure can greatly improve the utilization rate of Pt.
[0052] from image 3 As can be seen in , IrBlakck exhibits a typical Ir 3+ / Ir 4+ and Ir 4+ / Ir 5+ redox peak, while the IrBlakck surface-modified Pt-type catalyst prepared by ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com