Preparation method of tablet containing grease components at high dosage
A high-dose, oil-ester technology, applied in the directions of medical preparations containing active ingredients, medical preparations without active ingredients, and plant/algae/fungus/moss ingredients, etc., can solve the problem of low hardness, low tablet, tablet Fragmentation and edge knocking, etc., to achieve the effect of increasing hardness, improving brittleness and improving formability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
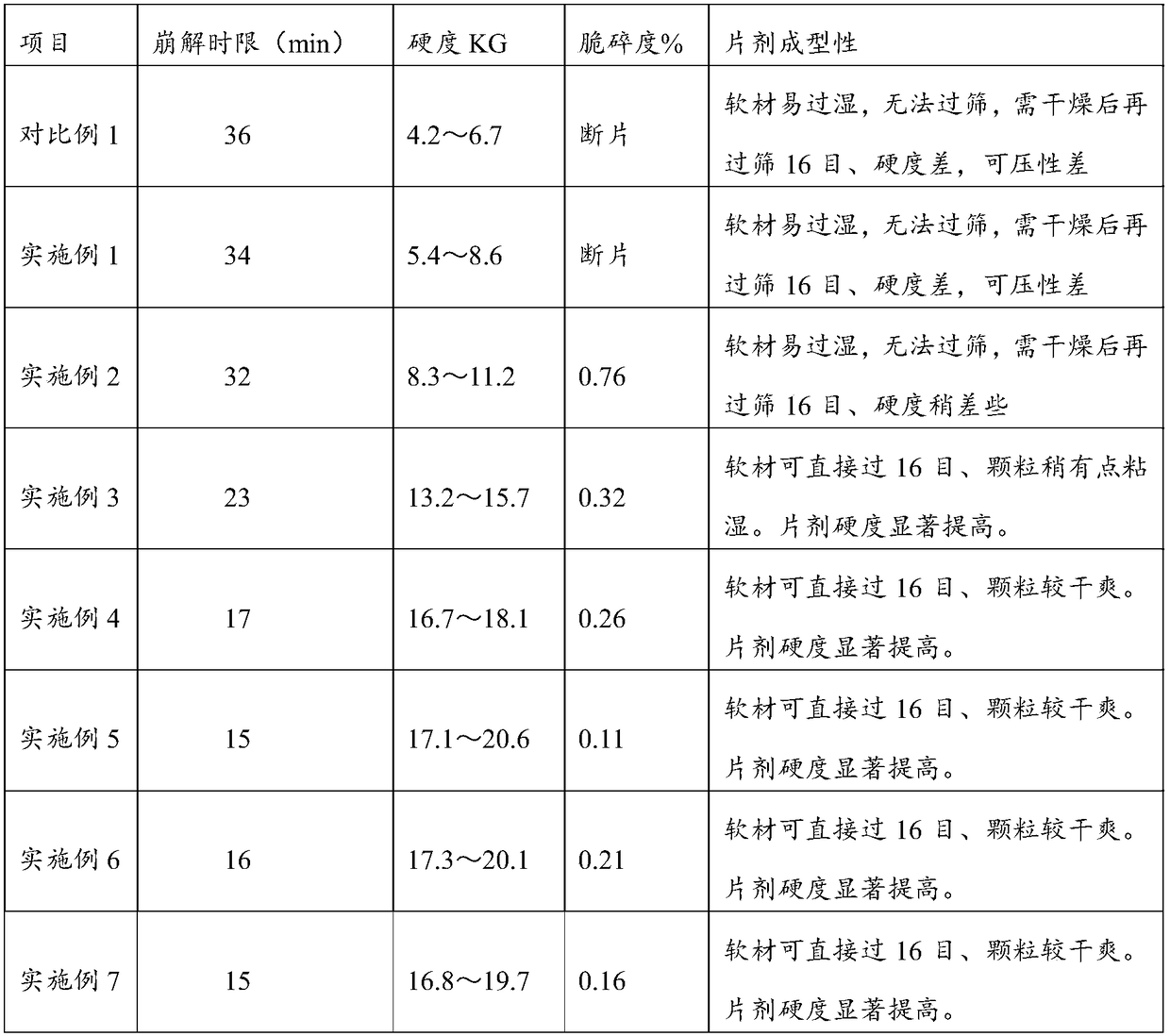
[0020] The invention provides a specific preparation method for tablets containing high-dose oily ester components, the specific steps comprising: mixing the oily ester active material (the oil ester in the oily ester active material accounts for 30%-50% by mass of the oily ester active material) %) and the first part of silicon dioxide into a wet granulator, mix until the color is uniform, add povidone K30 aqueous solution or povidone K30 ethanol solution to granulate while stirring, and the soft material made is over 16~ 20 mesh sieve, oven-dry the sieved granules at 40℃~45℃; after drying, use 16~20 mesh sieves for granulation, add the second part of silica, adsorbent and filler after granulation , disintegrant and slip material are mixed until the color is uniform. Tablet compression was performed using a rotary tablet press.
[0021] Wherein, the oily ester active substance is a powder.
[0022] Specifically, the active oily ester is powdered phytosterol ester.
[0023]...
Embodiment 1
[0028] The phytosterol ester powder that is 30% by mass percentage (wherein, sterol ester accounts for 50% by mass percentage of phytosterol ester powder) and the silicon dioxide that is 1% by mass percentage are placed in wet granulator, add while stirring The povidone K30 aqueous solution with a mass percentage of 6% is granulated, and the soft material made is passed through a 18-mesh sieve, and the sieved granules are dried in an oven at 43°C until the granules contain 3% moisture, and then used for 18 mesh sieve for granulation; after granulation granulation, adding mass percentage is 12% functional red yeast rice powder, mass percentage is 9.5% water-soluble tomato concentrate, mass percentage is 1% silicon dioxide, mass percentage is 20% Maltodextrin, mass percent is 16% microcrystalline cellulose, mass percent is 4% crospovidone, and mass percent is 0.5% magnesium stearate, the sample of the prepared embodiment 1 is detected for its disintegration Time limit, hardness,...
Embodiment 2
[0030] The phytosterol ester powder that is 30% by mass percentage (wherein, sterol ester accounts for 50% by mass percentage of phytosterol ester powder) and the silicon dioxide that is 1% by mass percentage are placed in wet granulator, add while stirring The povidone K30 aqueous solution with a mass percentage of 6% is granulated, and the soft material made is passed through a 18-mesh sieve, and the sieved granules are dried in an oven at 43°C until the granules contain 3% moisture, and then used for 18 mesh sieve for granulation; after granulation granulation, adding mass percentage is 10% functional red yeast rice powder, mass percentage is 7.5% water-soluble tomato concentrate, mass percentage is 1% silicon dioxide, mass percentage is 18% Calcium hydrogen phosphate, the microcrystalline cellulose that mass percent is 22%, the crospovidone that mass percent is 4%, the magnesium stearate that mass percent is 0.5%, the embodiment 2 sample that will make detects its disintegr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com