Fluorinated covalent organic polymer loaded with perfluorocarbon and its preparation method and application
A covalent organic, perfluorocarbon technology, applied in the field of medicine, can solve the problems of limiting the effect of photodynamic therapy substances, hypoxia at tumor sites, etc., to improve the hypoxic microenvironment of tumors, improve the effect, and improve the hypoxia The effect of the environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033]The present invention also provides a method for preparing a fluorinated covalent organic polymer that is filled with perfluorocarbon, including:
[0034]1) The carboxy-blocking polyethylene glycol, whole fluorocene and MESO-5-10-15-20-4 (4-hydroxyphenyl) porphyrin mixed to obtain a covalent organic polymer;
[0035]2) The fluorinated covalent organic polymer obtained by step 1) is mixed with allfluorocarbon to covalent organic polymers.
[0036]According to the present invention, the present invention reacts the carboxyl-terminated polyethylene glycol, whole fluorcyclic acid, and MESO-5-10-15-20-4 (4-hydroxyphenyl) porphyrin mixed to obtain a covalent organic polymerization The molar ratio of the MESO-5-10-15-20-4 (4-hydroxyphenyl) porphyrin and the whole fluorogenic acid is preferably 1: (1 to 2), more preferably 1: 1.5; the molar ratio of the MESO-5-10-15-20-4 (4-hydroxyphenyl) porphyrin and the carboxyl block of polyethylene glycol is 1: (1 to 4), more preferably 1: 2 ~ 3); the mas...
Embodiment 1
[0041]Example 1 Preparation of fluorinated fluorinated organic polymers (PFC @ THPP-PFSEA) loading perfluorocarbon
[0042]Meso-5-10-15-20-4 (4-hydroxyphenyl) porphyrin (0.04 mmol) (THPP) was dissolved in 50 ml of super dry tetrahydrofuran solution in wholefluorocetic acid (0.06 mmol), and then added to the second Cyclohexyl carbon diimide (0.8 mmol) and 4-dimethylaminopyridine (0.8 mmol), the oil bath is heated to 45 degrees Celsius, and the protected light reaction is 24 hours. Further, in the reaction system, a carboxyl-terminated polyethylene glycol (0.16 mmol) was then reacted at 45 ° C for 24 hours, and the reaction was terminated. In order to purify the MesO-5-10-15-20-4 (4-hydroxyphenyl) porphyrin and PFSea small molecules in the product, the reaction solvent tetrahydrofuran is removed by a rotary evaporator, and a small amount of methanol is dissolved and the reaction product is added and a large amount of ice. EtOAc (EtOAc) EtOAc. Ultra-pure water was dialyzed under room temp...
Embodiment 2
[0046]The fluorinated covalent organic polymers obtained in Example 1 were characterized (including: UV - visible absorption spectrum, dynamic light scattering, transmission electron microscopy, single-line oxygen production capability),Figure 1 ~ 5;
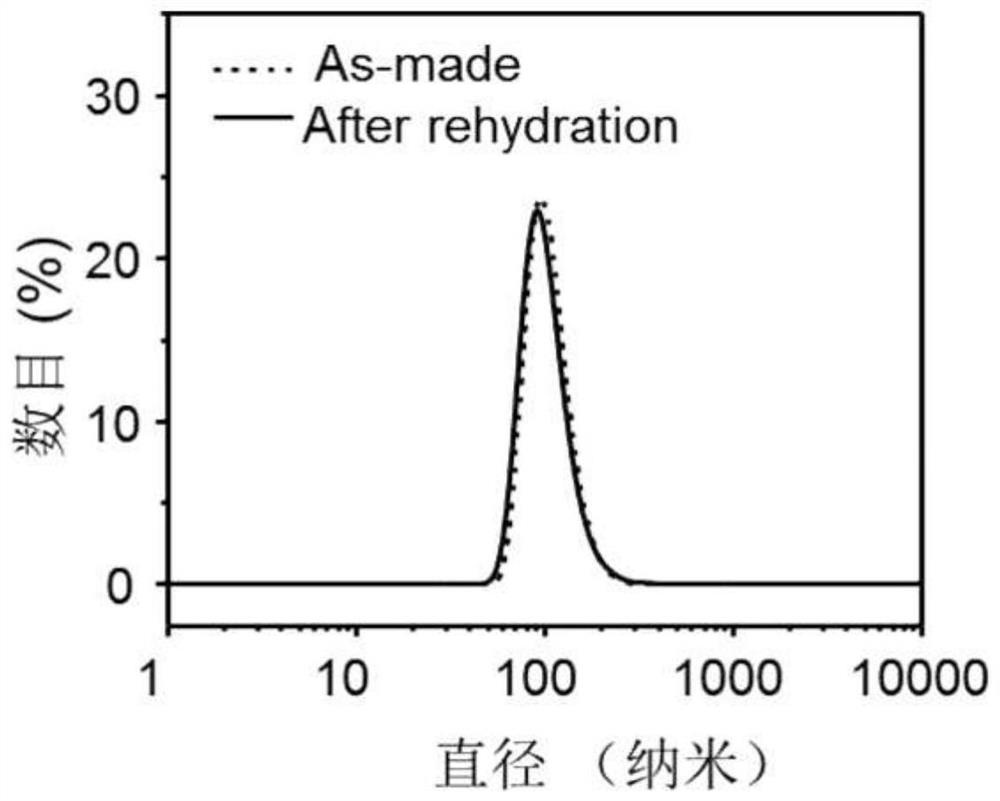
[0047]figure 1 The dynamic laser particle size diagram of the ThPP-PFSEA nanoparticles described in Example 1 was in water before and after hydration. As can be seen from the figure, the particle size before and after hydration has no significant change, indicating that the complex can be prepared into a powder product after lyophilization, facilitating long-term storage and transportation.
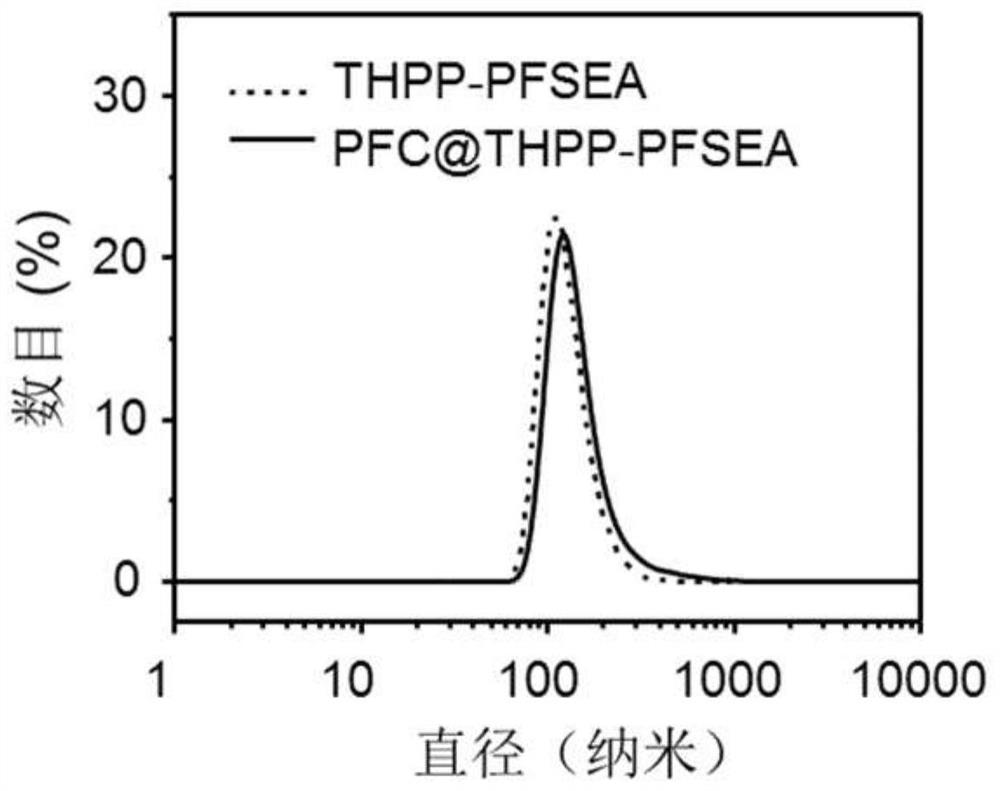
[0048]figure 2 The dynamic laser particle size distribution of ThPP-PFSEA nanoparticles described in Example 1 was loaded with full fluorocarbon. As can be seen from the figure, the nanoparticles formed by Thpp-PfSea are about 80 nm, and the particle size remains stable after loading perfluorocarbon.
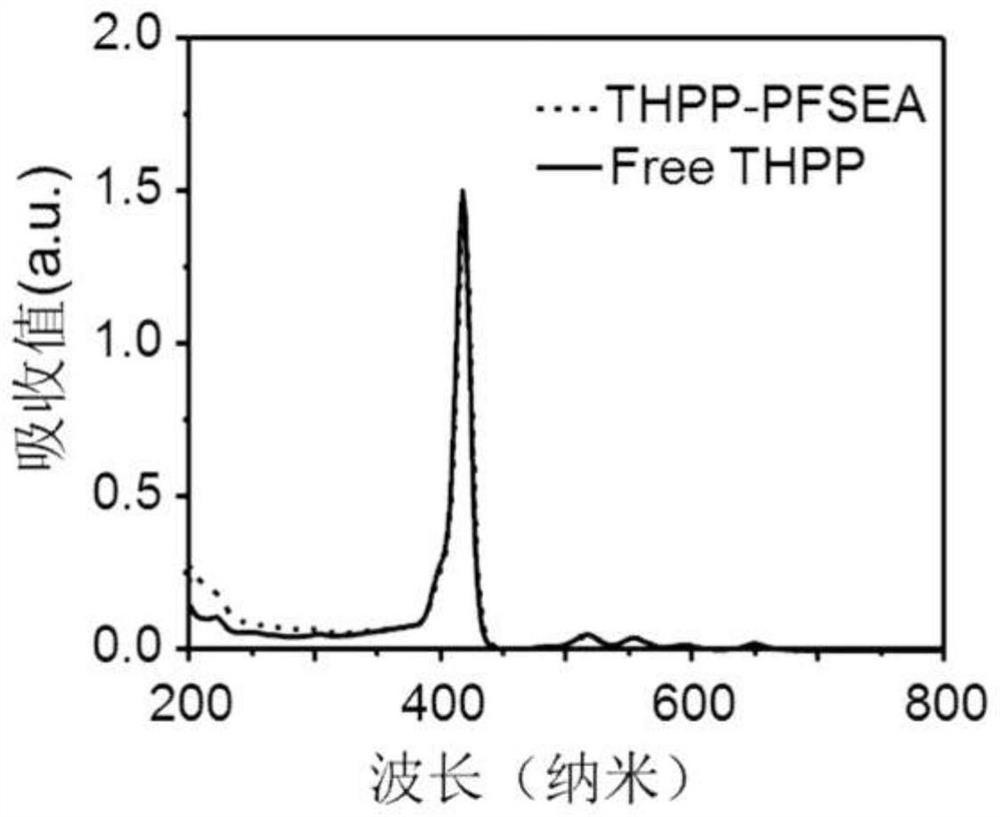
[0049]image 3 For the ultraviolet-visible absorption ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com