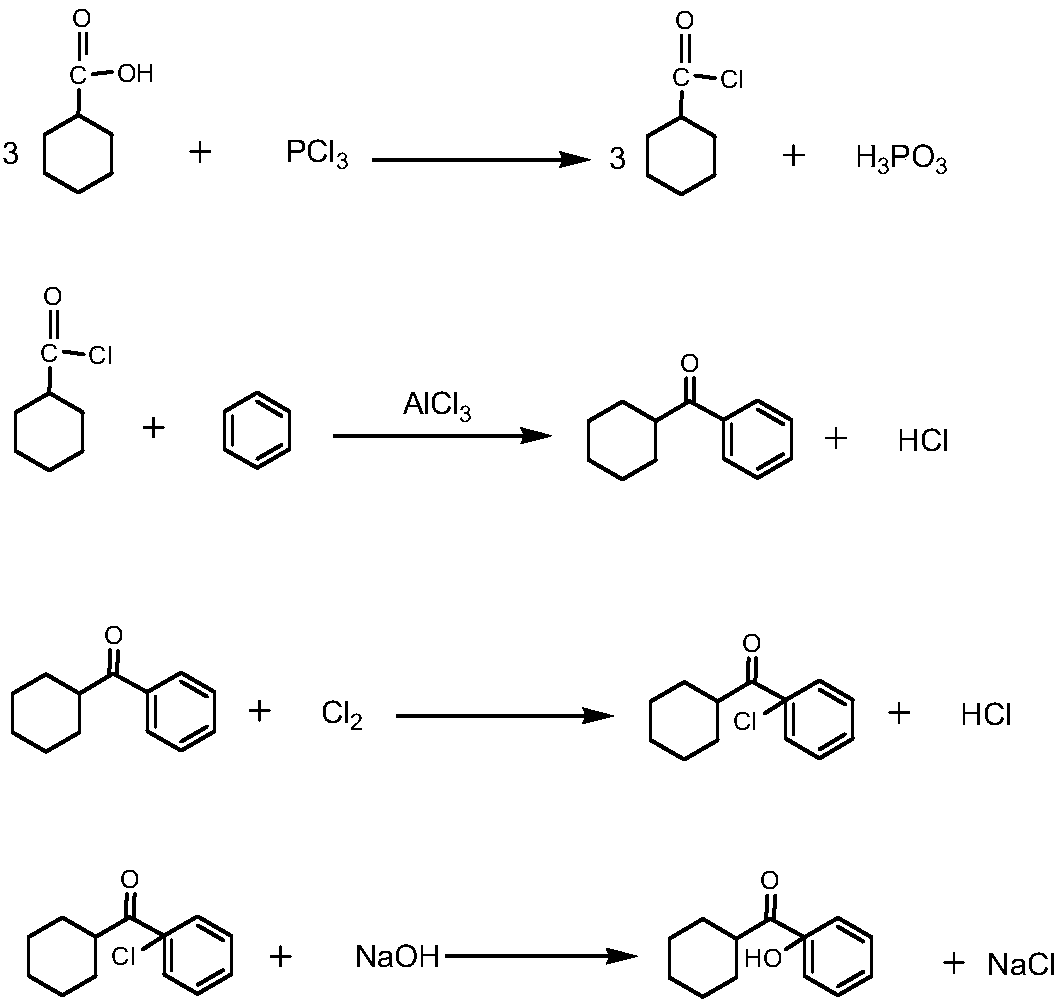
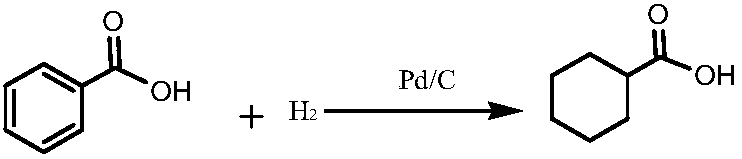Preparation method of photoinitiator 1-hydroxycyclohexyl benzophenone
The technology of hydroxycyclohexyl phenyl ketone and photoinitiator is applied in the field of preparation of photoinitiator 1-hydroxycyclohexyl phenyl ketone, and can solve the problems of large environmental pollution, high cost, low production efficiency and the like, Achieving the effect of avoiding environmental pollution, high yield and purity, and increasing production income
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] A preparation method of photoinitiator 1-hydroxycyclohexyl phenyl ketone, comprising the following steps,
[0020] S1, Reduction: pump a proportioned amount of liquid cyclohexanecarboxylic acid into the high-level metering tank, turn on the vacuum pump of the dissolution kettle to maintain a slight negative pressure state, put in benzoic acid, seal, stop the vacuum pump, and put cyclohexanecarboxylic acid in the metering tank In the kettle, heat up to dissolve, add catalyst to the dissolved solution, then replace the air in the hydrogenation kettle with nitrogen, and introduce hydrogen into the kettle when the temperature rises to 78°C until no hydrogen is absorbed, and the sampling is qualified Finally, lower the temperature to 35°C, pass the residual hydrogen in the kettle through the pressure relief buffer tank and the lye washing tank, and control the flow to empty it. After the hydrogen is exhausted to a slight positive pressure, it is completely replaced by nitroge...
Embodiment 2
[0027] A preparation method of photoinitiator 1-hydroxycyclohexyl phenyl ketone, comprising the following steps,
[0028] S1, Reduction: pump a proportioned amount of liquid cyclohexanecarboxylic acid into the high-level metering tank, turn on the vacuum pump of the dissolution kettle to maintain a slight negative pressure state, put in benzoic acid, seal, stop the vacuum pump, and put cyclohexanecarboxylic acid in the metering tank In the kettle, heat up to dissolve, add catalyst to the dissolved solution, then replace the air in the hydrogenation kettle with nitrogen, and introduce hydrogen into the kettle when the temperature rises to 80°C until no hydrogen is absorbed, and the sampling is qualified Finally, lower the temperature to 38°C, pass the residual hydrogen in the kettle through the pressure relief buffer tank and the lye washing tank, and control the flow to empty it. After the hydrogen is discharged to a slight positive pressure, it is completely replaced by nitrog...
Embodiment 3
[0035] A preparation method of photoinitiator 1-hydroxycyclohexyl phenyl ketone, comprising the following steps,
[0036]S1, Reduction: pump a proportioned amount of liquid cyclohexanecarboxylic acid into the high-level metering tank, turn on the vacuum pump of the dissolution kettle to maintain a slight negative pressure state, put in benzoic acid, seal, stop the vacuum pump, and put cyclohexanecarboxylic acid in the metering tank In the still, heat up to dissolve, add catalyst to the dissolved solution, then replace the air in the hydrogenation kettle with nitrogen, and introduce hydrogen into the kettle when the temperature rises to 82°C until no hydrogen is absorbed, and the sampling is qualified Finally, the temperature is lowered to 40°C, and the residual hydrogen in the kettle is emptied through the pressure relief buffer tank and the lye washing tank, and the flow is controlled. After the hydrogen is exhausted to a slight positive pressure, it is completely replaced by ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com