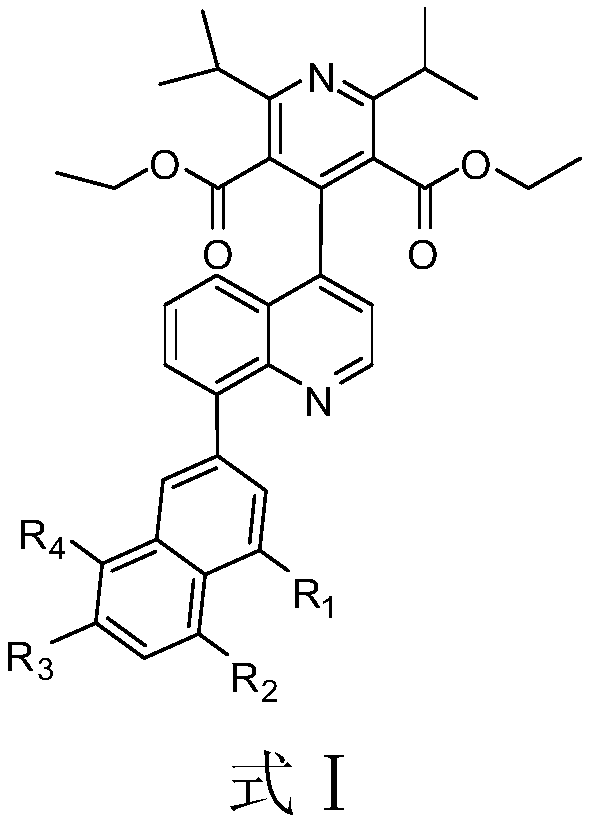
Quinoline derivative and application thereof in diabetes
A derivative, quinoline technology, applied in the field of quinoline derivatives and its application in diabetes, can solve the problems of inactivation and short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
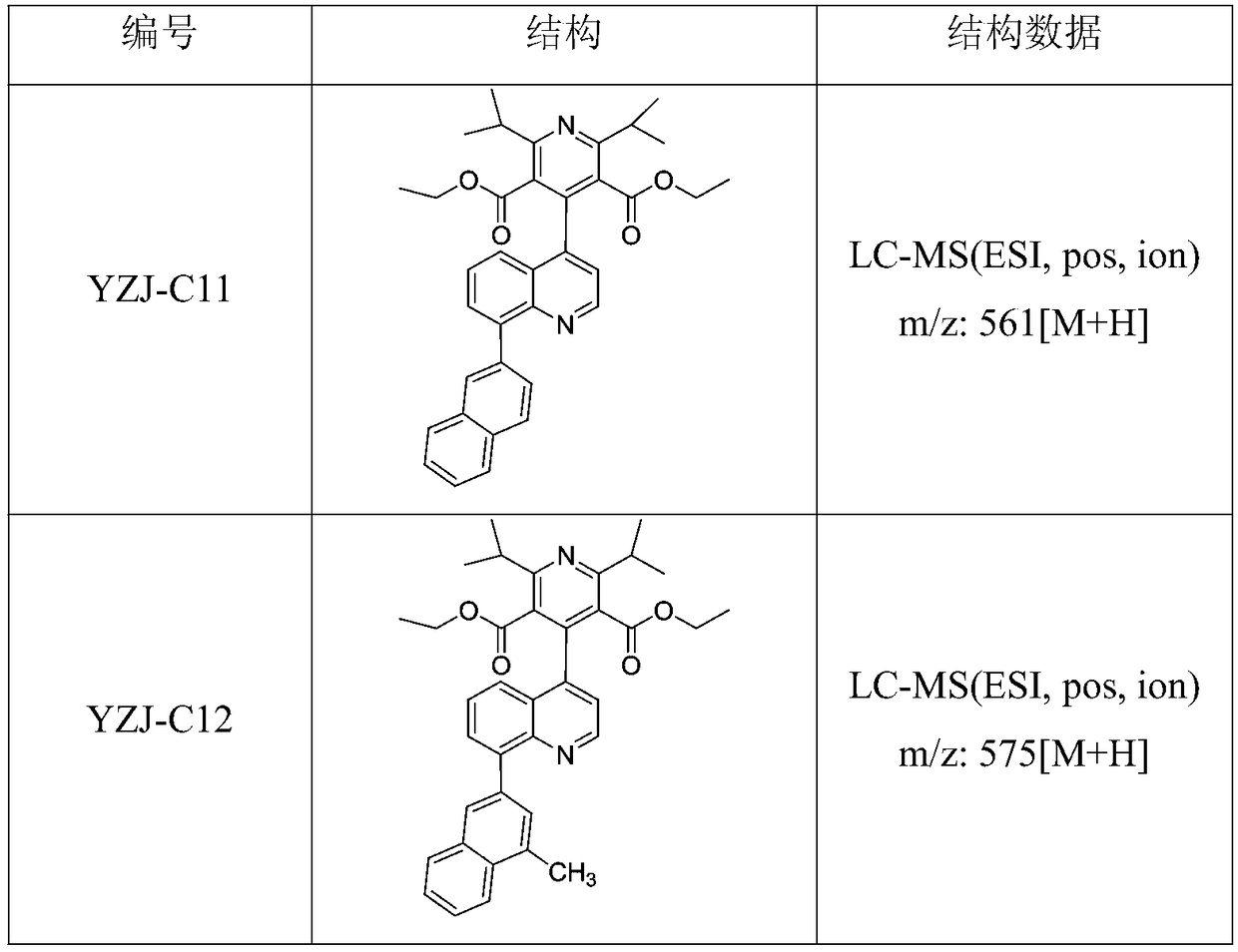
[0031] Example 1: Synthesis of 4-(8-(naphthalene-2-yl)-quinolin-2-yl)-2,6-diisopropylpyridine-3,5-dicarboxylic acid diethyl ester
[0032]
[0033] 1. Synthesis of (Z)-2-((8-bromo-quinolin-2-yl)methylene)-4-methyl-3-oxopentanoic acid ethyl ester
[0034]
[0035] In a 150 mL round bottom flask equipped with a magnetic stirrer, 8-bromo-quinoline-4-carbaldehyde (compound 1) (8.50 g, 36.00 mmol), ethyl 4-methyl-3-oxopentanoate ( Compound 2) (5.70 g, 36.03 mmol), isopropanol (21.1 mL), piperidine (2.2 mL) and glacial acetic acid (1.2 mL). The reaction mixture was stirred at room temperature under nitrogen for 12 hours. The solvent was removed under reduced pressure. The residue was dissolved with dichloromethane (13.88 mL) and washed with saturated NaHCO 3 (3*30mL) solution washed with anhydrous MgSO 4 After drying, remove MgSO by filtration 4 , after concentrating the solvent under reduced pressure, flash column chromatography to obtain off-white solid (Z)-2-((8-bromo-...
Embodiment 2
[0045] Example 2: 4-(8-(4-methyl-naphthalen-2-yl)-quinolin-2-yl)-2,6-diisopropylpyridine-3,5-dicarboxylic acid diethyl ester synthesis
[0046]
[0047] 4-methyl-naphthalene-2-boronic acid (19.60mmol), compound 5 (9.69g, 18.87mmol), Na 2 CO 3 (6.23g, 58.8mmol), DME (31.75mL) and H 2 O (7.83 mL) was added to a 100 mL microwave vial. vial with N 2 Degas for 1 h, then add PdCl 2 (dppf)CH 2 Cl 2 (1.73 g, 2.35 mmol) adduct. The reaction mixture was heated at 120 °C for 2 hours by microwave irradiation. The resulting mixture was diluted with ethyl acetate and filtered through celite, then concentrated in vacuo. Purification by flash chromatography using 0-100% ethyl acetate / heptane as eluent afforded 4-(8-(4-methyl-naphthalen-2-yl)-quinolin-2-yl as an off-white solid )-diethyl 2,6-diisopropylpyridine-3,5-dicarboxylate, 8.57 g, yield 79%. LC-MS (ESI, pos, ion) m / z: 575 [M+H].
Embodiment 3
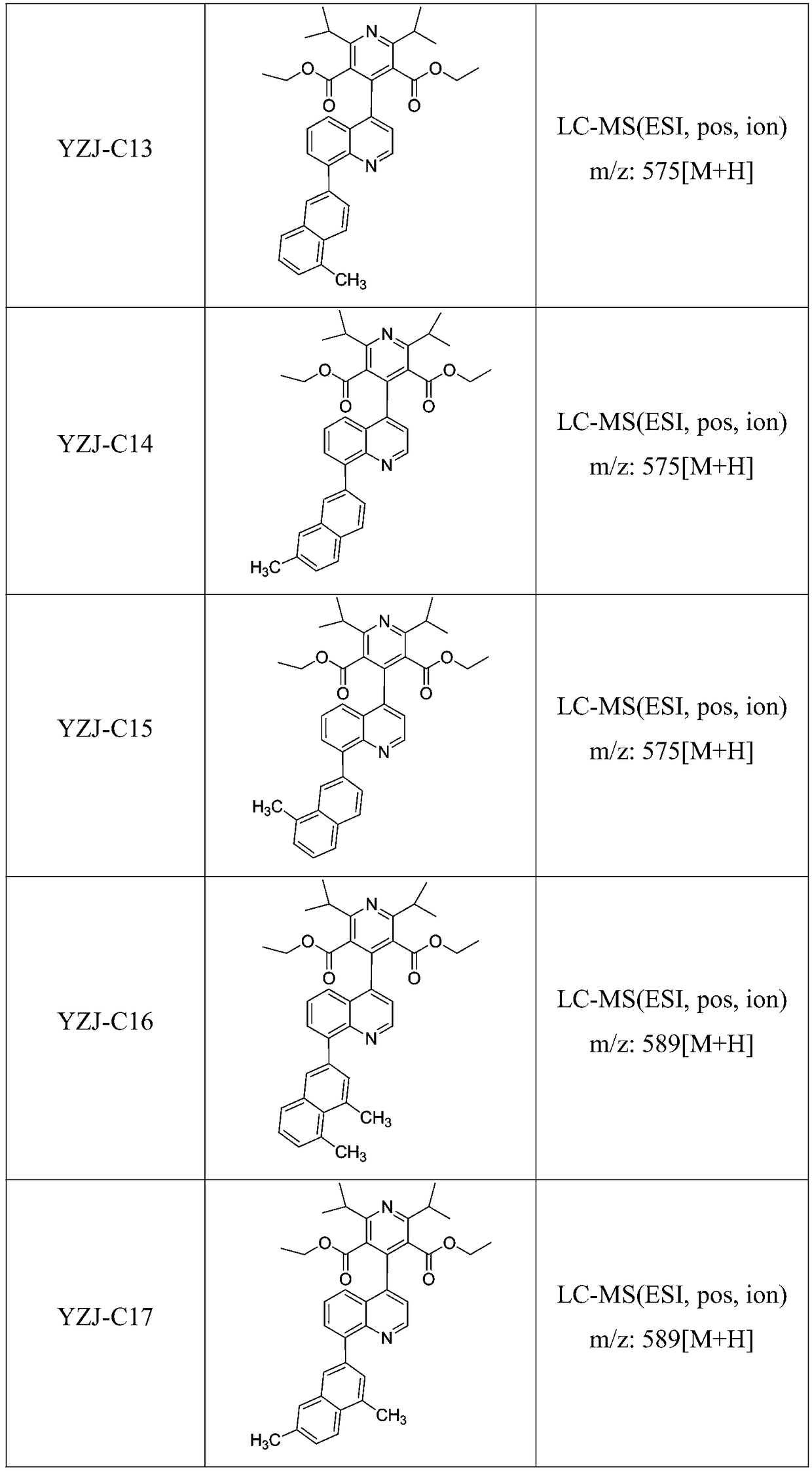
[0048] Example 3: 4-(8-(4,5 Dimethyl-naphthalen-2-yl)-quinolin-2-yl)-2,6-diisopropylpyridine-3,5-dicarboxylic acid diethyl Synthesis of esters
[0049]
[0050] 4,5 dimethyl-naphthalene-2-boronic acid (19.60mmol), compound 5 (9.69g, 18.87mmol), Na 2 CO 3 (6.23g, 58.8mmol), DME (31.75mL) and H 2 O (7.83 mL) was added to a 100 mL microwave vial. vial with N 2 Degas for 1 h, then add PdCl 2 (dppf)CH 2 Cl 2 (1.73 g, 2.35 mmol) adduct. The reaction mixture was heated at 120 °C for 2 hours by microwave irradiation. The resulting mixture was diluted with ethyl acetate and filtered through celite, then concentrated in vacuo. Purification by flash chromatography using 0-100% ethyl acetate / heptane as eluent afforded 4-(8-(4,5-dimethyl-naphthalen-2-yl)-quinoline-2 as an off-white solid -Diethyl)-2,6-diisopropylpyridine-3,5-dicarboxylate, 8.44 g, yield 76%. LC-MS (ESI, pos, ion) m / z: 589 [M+H].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com