Test card for rapid detection of epidemic hemorrhagic fever virus antibody, and application of test card
An epidemic hemorrhagic fever, detection and testing technology, applied in the field of immune detection, can solve problems such as inconvenience and time-consuming, achieve high accuracy, avoid false negative results, and facilitate field detection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
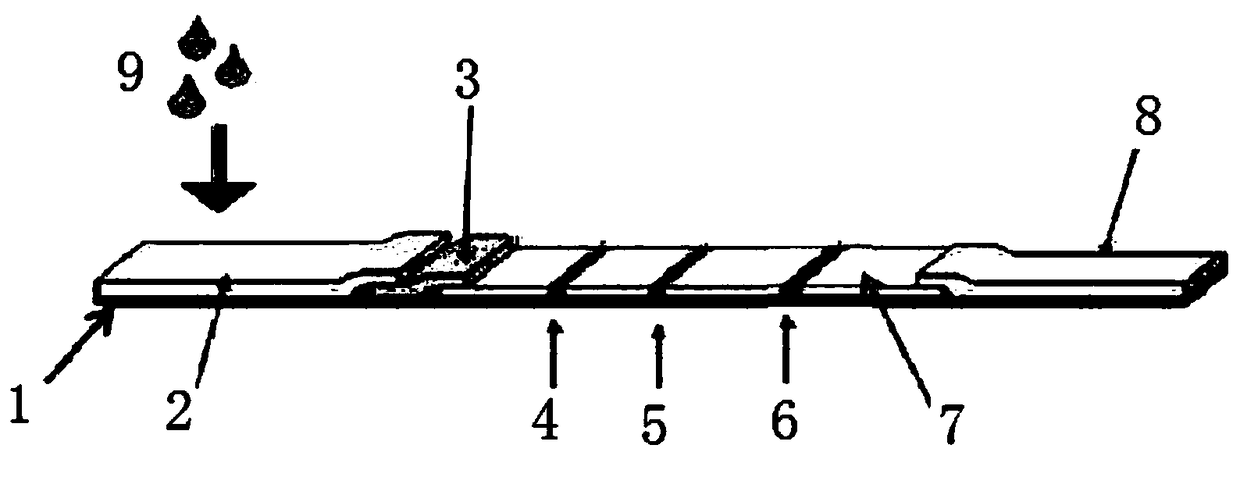
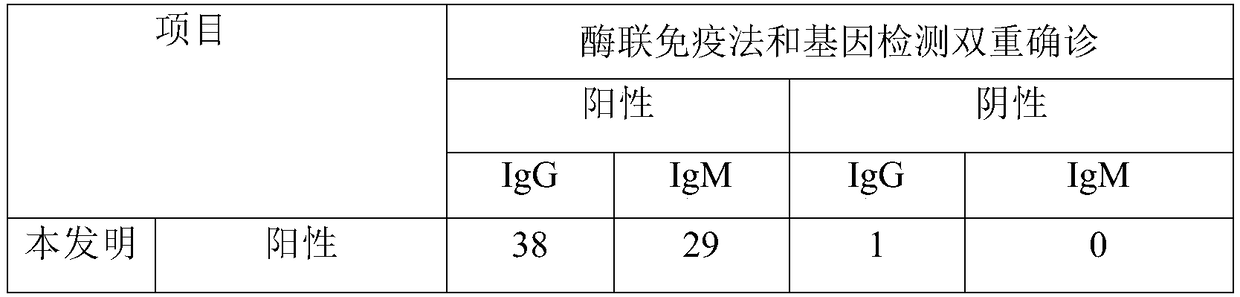
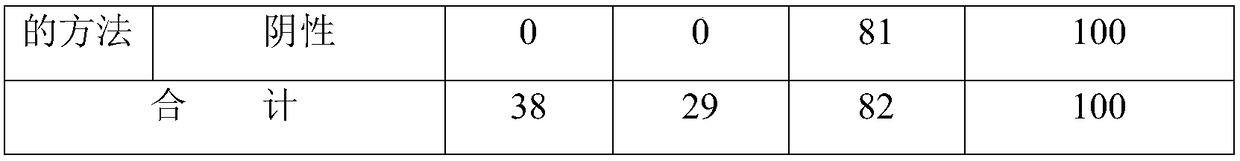
Image
Examples
Embodiment 1
[0029] The determination of embodiment 1 epidemic hemorrhagic fever virus antigen, anti-human IgG antibody and anti-human IgM antibody
[0030]According to the design of the inventor, the antigen of the amino acid sequence shown in SEQID NO: 1 has been designed from a large amount of antigens of epidemic hemorrhagic fever (EHF) virus as the EHF virus detection antigen; in addition, the inventor has found that commercially available Anti-human antibodies are prone to certain cross-reactions with the above-mentioned detection antigens, resulting in a decrease in detection accuracy. Therefore, two antigens (whose amino acid sequences are shown in SEQ ID NO: 2 and 3 respectively) have been screened out from a large number of human IgG and IgM. Shown), effective anti-human IgG antibody and anti-human IgM antibody can be prepared without complete human IgG and IgM respectively, entrusted Beijing Zhongshan Jinqiao Biotechnology Co., Ltd. to prepare the amino acid sequence shown in SEQ...
Embodiment 2
[0031] Embodiment 2 anti-human IgG, anti-human IgM monoclonal antibody draws film on nitrocellulose
[0032] The anti-human IgG antibody and the anti-human IgM antibody prepared in Example 1 were used to draw a film on a nitrocellulose membrane (NC membrane) with a nitrocellulose membrane, specifically using a Sartori μs (Sartorius) CN104NC membrane with a pore size of 15 μm and a crawling speed of 150S / 4cm. The specific filming process is as follows:
[0033] 1) Preparation of scratching buffer: prepare 0.01 MPBS (phosphate buffer), pH 7.0, containing 1% sucrose, and filter with 0.22 μm.
[0034] 2) Take the scratching buffer and dilute the anti-human IgG antibody, anti-human IgM antibody and anti-rabbit antibody (Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.) respectively according to a certain concentration, wherein the anti-human IgG antibody is diluted to 2.5mg / ml, and the anti-human IgG antibody The IgM antibody was diluted to 1.0mg / ml, and the anti-rabbit antibody...
Embodiment 3
[0036] The determination of embodiment 3 fluorescent microspheres and the coupling with antigen, antibody
[0037] Take carboxyl group fluorescent microspheres from different sources, dilute them with 0.01M PBS at 1:10; use 0.01M PBS to adjust to zero, and scan in the wavelength range of 400nm to 600nm to see if there is a narrow absorption peak at a wavelength of 480nm (indicating fluorescence Uniform microsphere particle size); Dilute the concentration of fluorescent microspheres to 2mg / ml with 0.01M PBS, spot 10μl on the NC membrane, and check to see if the fluorescence value is ≥10 4 . After testing, the fluorescent microspheres with carboxyl groups purchased from Ocean Nanotech have the best effect, so this fluorescent microspheres were selected. The specific antigen and antibody coupling process is as follows:
[0038] 1) EHF antigen-labeled fluorescent microspheres: Dilute the fluorescent microspheres to 1 mg / ml with activation buffer (0.05M MES (2-(N-morpholino)ethan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com