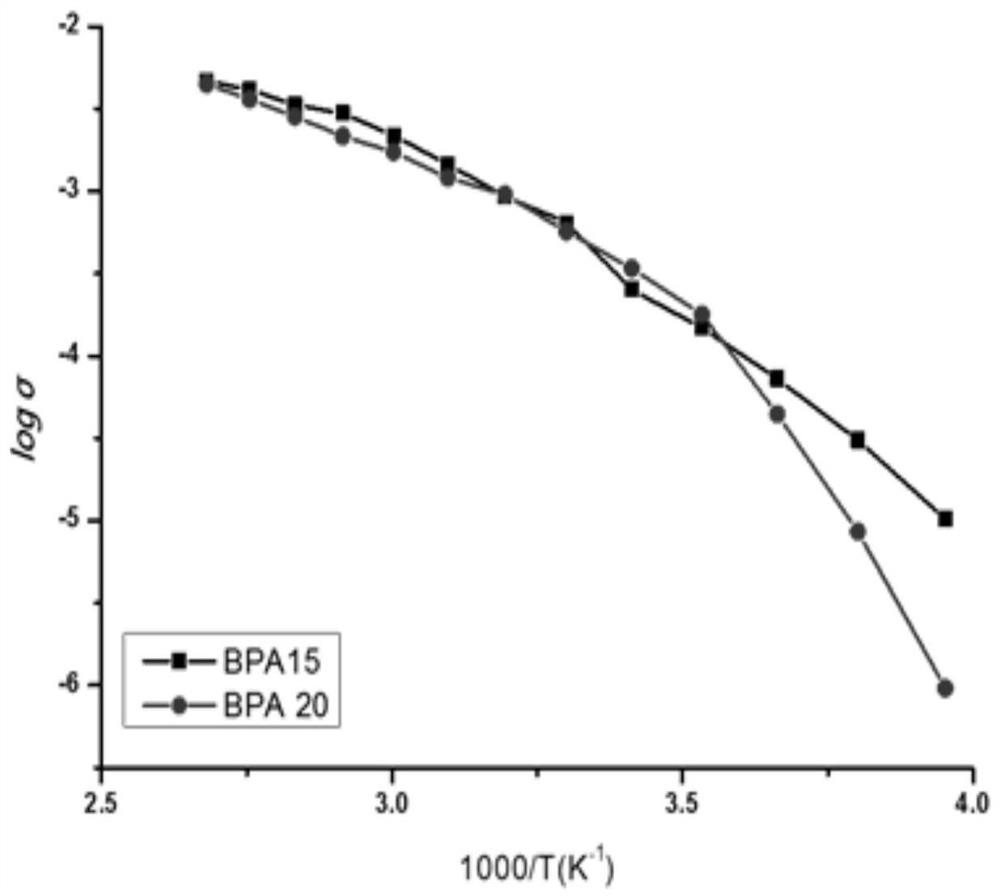
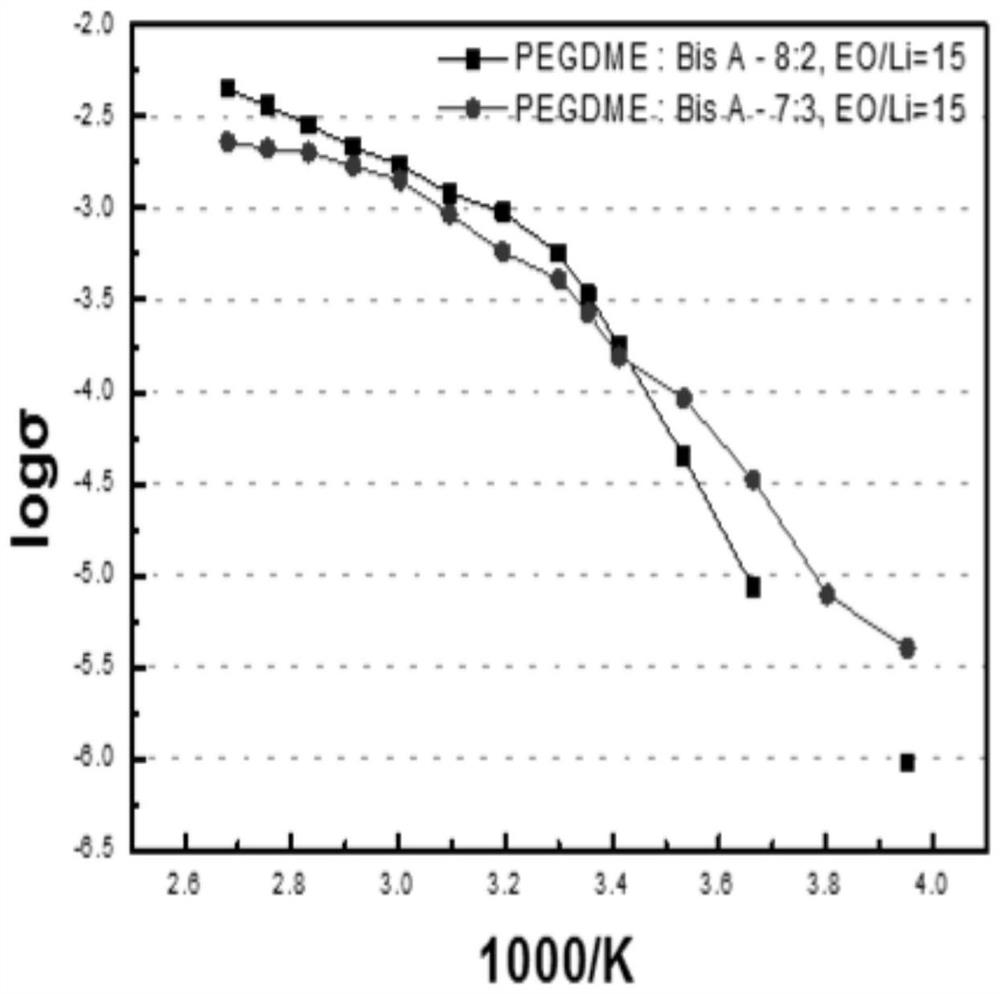
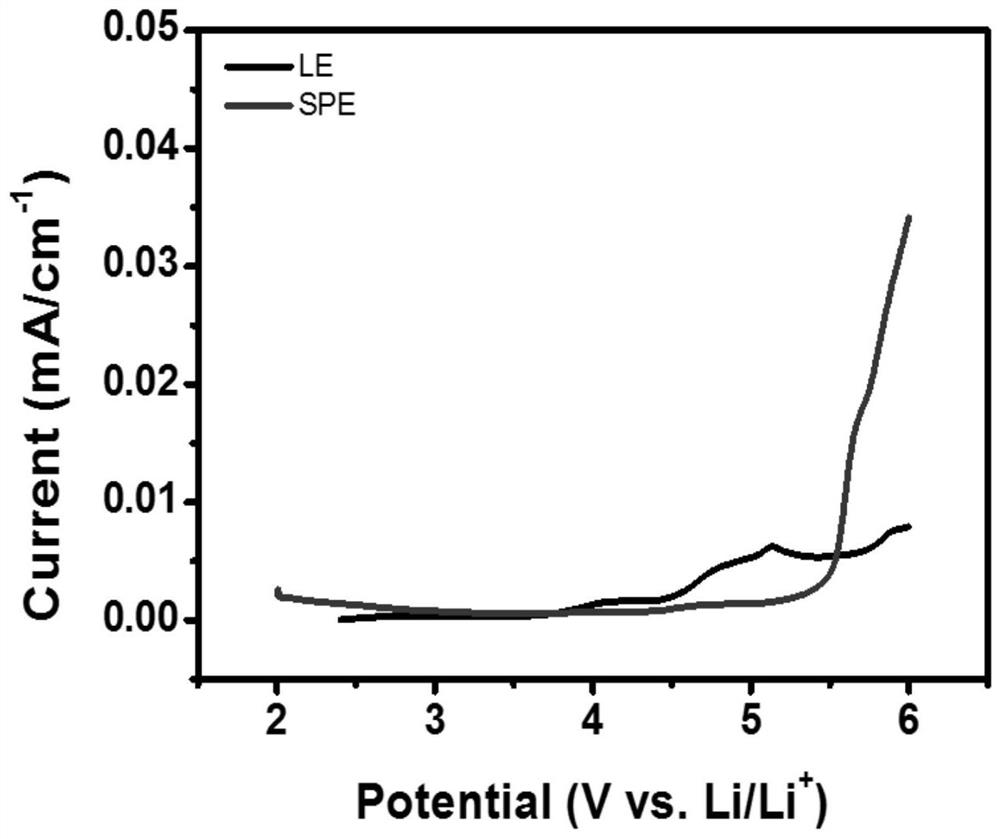
A kind of solid polymer electrolyte and its preparation method and lithium ion secondary battery
A solid polymer, electrolyte technology, applied in solid electrolyte, secondary battery, non-aqueous electrolyte and other directions, can solve the problem of low capacity of lithium ion secondary battery, and achieve easy industrial production, low cost, simple and convenient preparation process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The present invention also relates to a method for preparing the above-mentioned solid polymer electrolyte, comprising the following steps:
[0027] S1, preparing polytrimethylene carbonate by polymerization of trimethylene carbonate;
[0028] S2. Mix polytrimethylene carbonate, plasticizer and lithium salt to prepare a first mixed solution;
[0029] S3, then add the bridging agent to the first mixed solution, and mix evenly to prepare the second mixed solution;
[0030] S4. Adding the second initiator to the second mixed solution and heating to obtain a solid polymer electrolyte.
[0031] In the above-mentioned embodiment, the solvent is methanol, and the specific processes of steps S2, S3 and S4 can adopt the prior art. Preferably, in step S1, trimethylene carbonate and initiator are added to the ampoule, which is immersed in liquid nitrogen In , after vacuum sealing the ampoule, the ampoule was heated to polymerize the trimethylene carbonate.
[0032] The present ...
Embodiment 1
[0035] The preparation method of the polytrimethylene carbonate in the present embodiment, comprises the following steps:
[0036] 0.99g trimethylene carbonate (TMC) and 0.081ml diethylzinc (its weight ratio accounts for 1wt%) are packed into ampoule, it is immersed in liquid nitrogen, after utilizing vacuum pump to vacuum-seal the ampoule, at 60 Stir and heat at ℃ for 24 hours. After the reaction is over, add chloroform. After the unreacted monomer is dissolved, add methanol to precipitate under cooling, filter the precipitate, wash with methanol, and dry in vacuum to obtain polytrimethylene carbonate. (Poly trimethylene carbonate, PTMC), the production rate is 90%.
[0037] The molecular weight of the obtained PTMC is different when the dosage of the initiator is different. The results are shown in Table 2.
[0038] Table 2
[0039]
[0040]
Embodiment 2
[0042] The preparation method of the polytrimethylene carbonate in the present embodiment, comprises the following steps:
[0043] 3g of trimethylene carbonate (TMC) and 20 μl of stannous ethylhexanoate (0.0008wt% by weight) were packed into an ampoule, immersed in liquid nitrogen, and vacuum-sealed using a vacuum pump, the ampoule was Stir and heat at 130°C for 72 hours. After the reaction is over, add chloroform. After the unreacted monomers are dissolved, add methanol to precipitate under cooling, filter the precipitate, wash with methanol, and vacuum dry to obtain PTMC. The yield is 90%.
[0044] The molecular weight of the obtained PTMC is different when the dosage of the initiator is different, and the results are shown in Table 3.
[0045] table 3
[0046] Initiator (wt%) mn mw PDI (20μl, 0.0008wt%) 46,132 104,237 2.26 (100μl, 0.004wt%) 53,782 114,684 2.13
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com