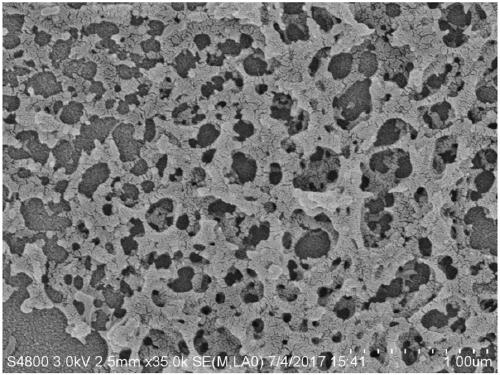
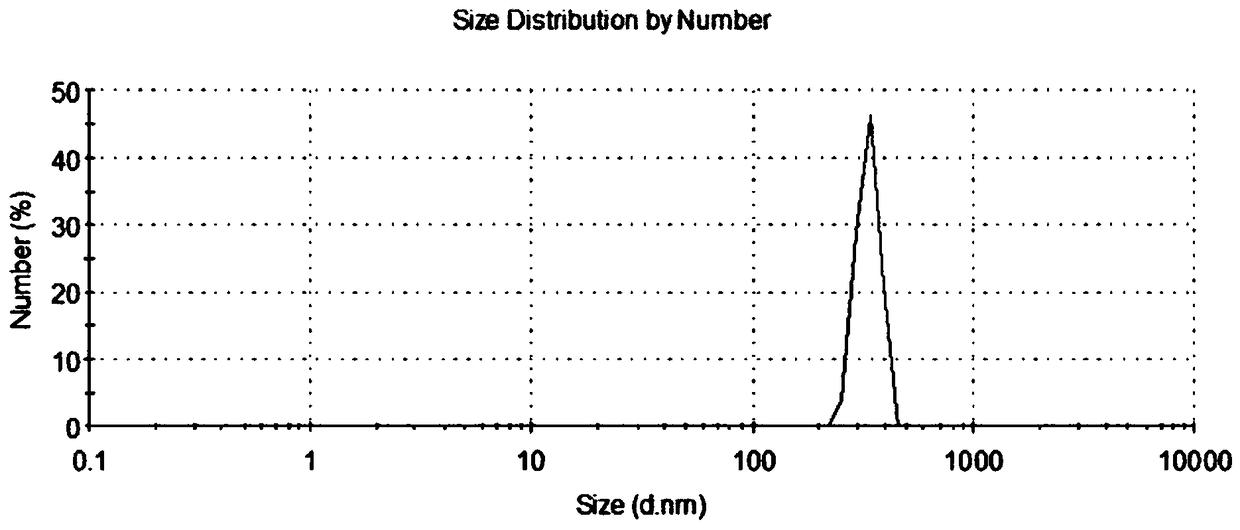
Modified chitosan-alginate-dragon's blood composite nano hemostatic material and preparation method thereof
A technology of alginate and hemostatic material, applied in pharmaceutical formulations, surgical adhesives, applications, etc., can solve problems such as unsatisfactory hemostatic effect, slow hemostasis, wound irritation, etc., to prolong the action time of the drug and improve the encapsulation rate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Preparation of Composite Nano Hemostatic Material with Wall Material and Core Material Mass Ratio of 1:1
[0049] 1. Preparation of carboxylated polyethylene glycol monomethyl ether
[0050] Dissolve 3.895g of mPEG with a molecular weight of 2000Da in 75ml of deionized water, then add 0.9925g of NaBr and 0.094g of TEMPO into the mPEG aqueous solution, after complete dissolution, add 46.75ml of NaClO to react at room temperature for 30min, then adjust the pH with 5M NaOH After reacting for 20 minutes, add HCl to adjust the pH to 1, then extract with an equal volume of chloroform several times, put it into 250ml of ice ethanol after vacuum distillation, recrystallize at -20°C for 12 hours, filter and dry to obtain carboxylated polymer Ethylene glycol monomethyl ether.
[0051] 2. Preparation of amphiphilic modified chitosan
[0052] Dissolve 0.1g chitosan and 0.1g HOBT in 50ml deionized water, stir vigorously to form a clear CS-HOBT solution, then add 0.2g N-a...
Embodiment 2
[0059] Example 2 Preparation of composite nano-hemostatic material with wall material and core material mass ratio of 1:3
[0060] 1. Preparation of carboxylated polyethylene glycol monomethyl ether
[0061] Dissolve 7.79g of mPEG with a molecular weight of 1000Da in 150ml of deionized water, then add 1.82g of NaBr and 0.18g of TEMPO into the mPEG aqueous solution, and after it is completely dissolved, add 86.91ml of NaClO to react at room temperature for 30min, then adjust with 5M NaOH The pH is 10.8. After reacting for 20 minutes, add HCl to adjust the pH to 1, then extract with an equal volume of chloroform several times, put it into 250ml of ice ethanol after vacuum distillation, recrystallize at -20°C for 12 hours, filter and dry to obtain carboxylation Polyethylene glycol monomethyl ether.
[0062] 2. Preparation of amphiphilic modified chitosan
[0063] Dissolve 0.5g chitosan and 0.5g HOBT in 100ml deionized water, stir vigorously to form a clear CS-HOBT solution, the...
Embodiment 3
[0069] Example 3 Preparation of Composite Nano Hemostatic Material with Wall Material and Core Material Mass Ratio of 1:5
[0070] 1. Preparation of carboxylated polyethylene glycol monomethyl ether
[0071] Dissolve 5.84g of mPEG with a molecular weight of 4000Da in 150ml of deionized water, then add 1.48g of NaBr and 0.141g of TEMPO into the mPEG aqueous solution, and after it is completely dissolved, add 70.12ml of NaClO to react at room temperature for 30min, then adjust with 5M NaOH The pH is 10.8. After reacting for 20 minutes, add HCl to adjust the pH to 1, then extract with an equal volume of chloroform several times, put it into 250ml of ice ethanol after vacuum distillation, recrystallize at -20°C for 12 hours, filter and dry to obtain carboxylation Polyethylene glycol monomethyl ether.
[0072] 2. Preparation of amphiphilic modified chitosan
[0073] Dissolve 0.3g chitosan and 0.3g HOBT in 100ml deionized water, stir vigorously to form a clear CS-HOBT solution, th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com