PRMT type-I inhibitor, and preparation method and application thereof
A compound and isomer technology, which is applied in the preparation of sulfides, amino compounds, chemical instruments and methods, etc., can solve the problems of off-target, poor cell membrane penetration, and no significant improvement in activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0129] The preparation of embodiment 1 PT-1 compound
[0130] Step i:
[0131]
[0132] Under nitrogen protection, o-chlorobenzaldehyde (14.6 grams, 1 equivalent), o-chlorothiophenol (30 grams, 2 equivalents) and potassium carbonate (43.8 grams, 3 equivalents) were added in DMF (200 milliliters) respectively, oil The bath was heated to 90°C overnight with stirring. All raw materials have been converted. Cool, pour into 2 liters of water, and extract 3 times with methyl tert-butyl ether. The combined organic phases were dried over anhydrous sodium sulfate, concentrated, and the crude product was separated by column chromatography to obtain 23.5 g of a yellow solid product.
[0133] Step ii:
[0134]
[0135] The raw material (15 g, 1 eq) was dissolved in 200 ml of anhydrous methanol, cooled to 0°C, and sodium borohydride (3 g, 1.3 eq) was added in portions. Warm to room temperature and continue stirring for 20 minutes. Cool down to 0°C, add saturated ammonium chlori...
Embodiment 2
[0145] The preparation of embodiment 2 PT-1A oxalate
[0146]
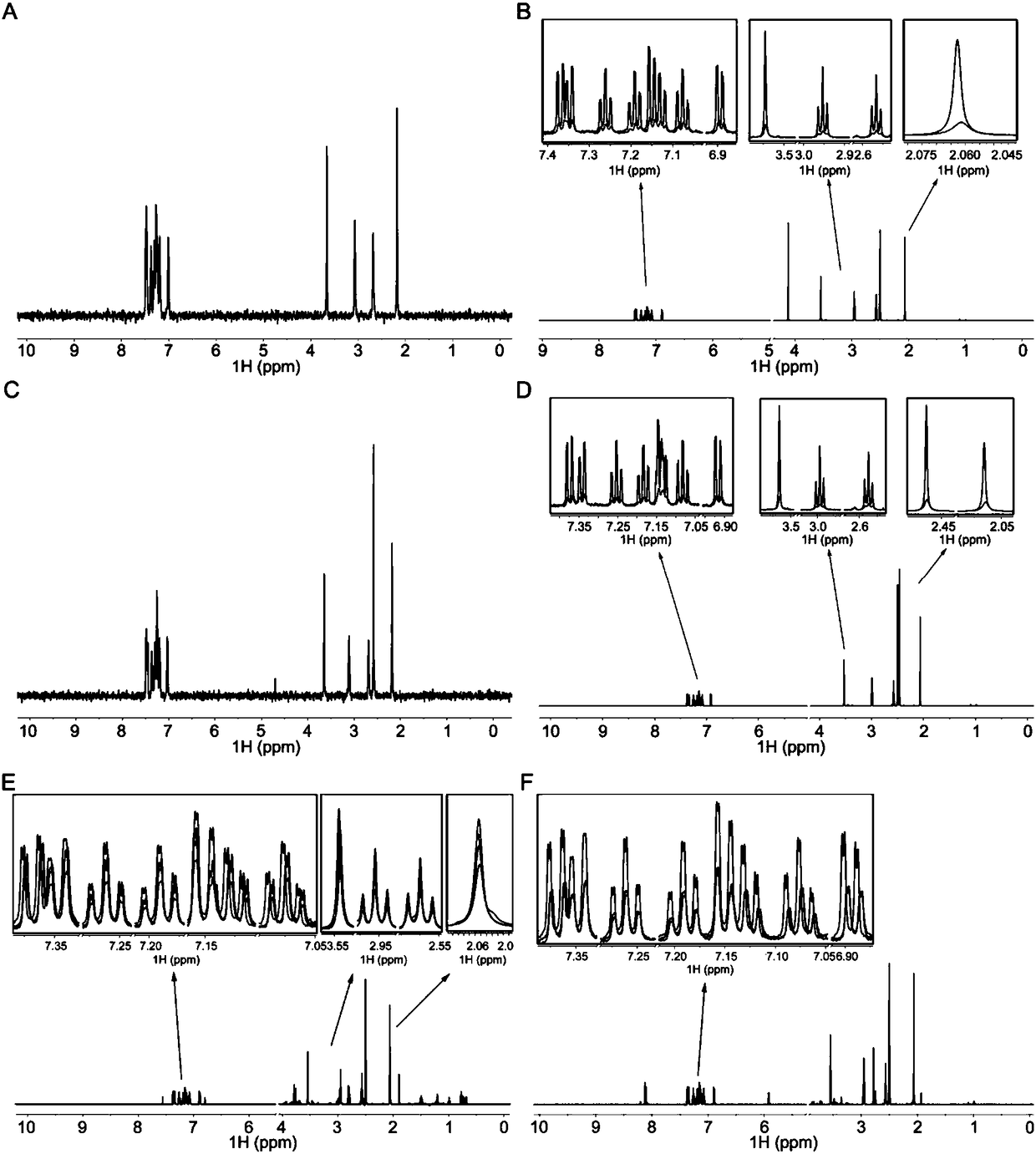
[0147] The amine (200 mg, 1 eq) was dissolved in 1 mL of ethyl acetate, and a solution of oxalic acid (59 mg, 1 eq) in ethyl acetate (3 mL) was added dropwise, a precipitate was formed, and stirring was continued for 30 minutes. Filter, wash with ethyl acetate, collect, dry to obtain 253 mg of white solid product, characterization data: 1 H NMR (400MHz, DMSO-d 6 )δ7.66(d, J=7.6Hz, 1H), 7.57–7.51(m, 1H), 7.42(d, J=7.5Hz, 1H), 7.36–7.21(m, 4H), 6.94–6.83(m , 1H), 3.63(s, 2H), 2.92(t, J=6.4Hz, 2H), 2.59(t, J=6.3Hz, 2H), 2.11(s, 3H). Purity: 98.7%.
Embodiment 3
[0148] The preparation of embodiment 3 PT-1B tartrate compound
[0149]
[0150] Amine (100 mg, 1 eq) was dissolved in 1 mL of ethyl acetate, a solution of tartaric acid (74 mg, 1.5 eq) in ethyl acetate (2 mL) and methanol (1 mL) was added dropwise, and stirred overnight at room temperature. The resulting white precipitate was collected by filtration, washed with ethyl acetate, and dried to give 123 mg of the product as a white solid. Characterization data: 1 H NMR (400MHz, DMSO-d 6 )δ7.66(d, J=7.6Hz, 1H), 7.57–7.51(m, 1H), 7.42(d, J=7.5Hz, 1H), 7.36–7.21(m, 4H), 6.94–6.83(m , 1H), 3.96(s, 3H), 3.63(s, 2H), 2.92(t, J=6.4Hz, 2H), 2.59(t, J=6.3Hz, 2H), 2.11(s, 3H). Purity: 98.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com