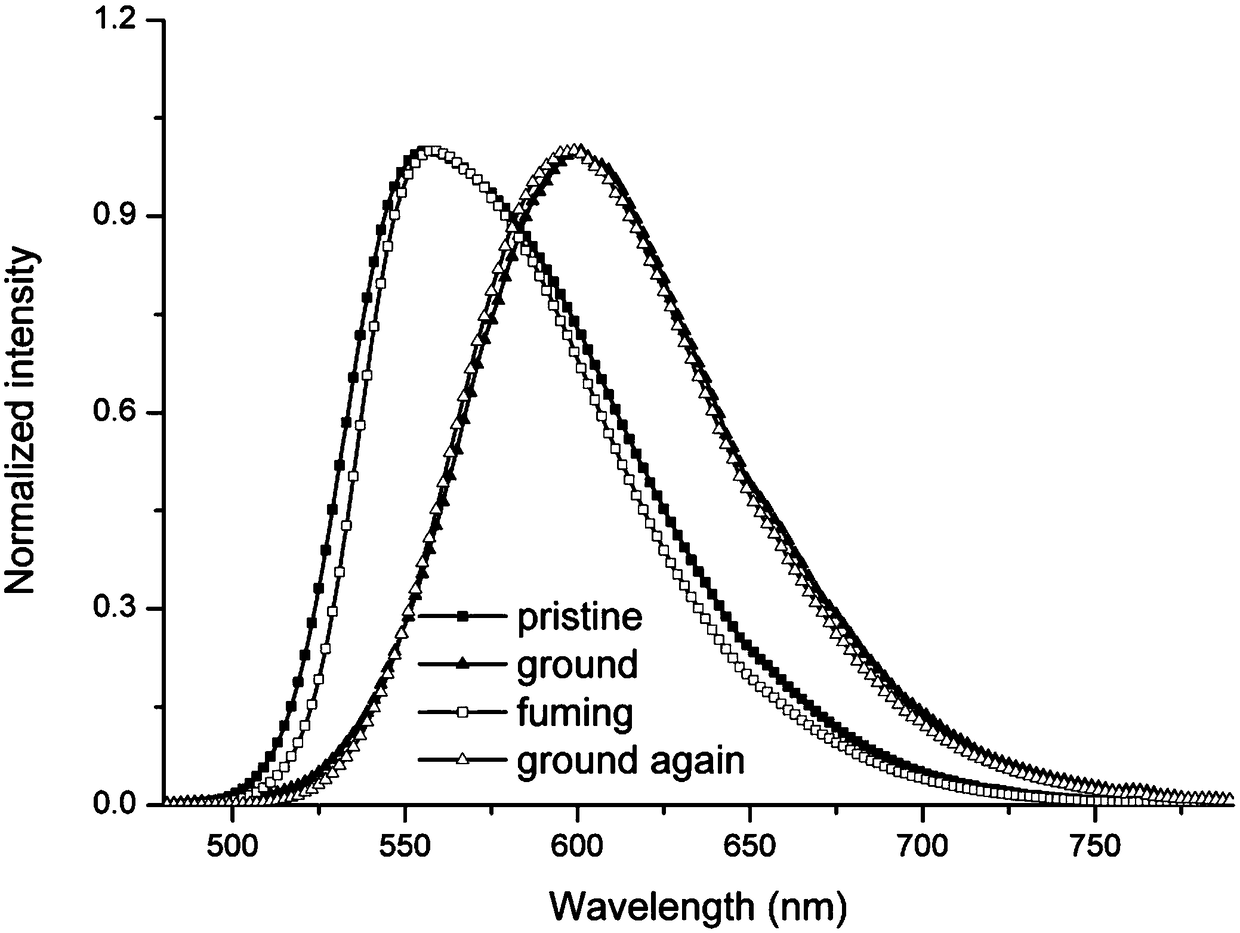
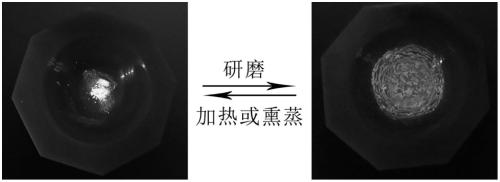
Mechanochromic diethyl terephthalate compound, as well as preparation method and application thereof
A technology of diethyl terephthalate and diethyl phthalate, which is applied in the field of diethyl terephthalate compounds and their preparation, can solve problems such as the expansion of functional materials, achieve good optical stability, and prepare Simple method, high reproducibility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Take 10g of 2,5-dibromoterephthalic acid in a 100mL round-bottomed flask, add 20mL of thionyl chloride under stirring, heat to reflux for 10 hours, and distill off excess thionyl chloride under normal pressure to obtain the corresponding acid chloride. Slowly add 10 mL of absolute ethanol dropwise under cooling at 0°C, after the dropwise addition is complete, stir at room temperature for 6 hours, evaporate the solvent to dryness to obtain diethyl 2,5-dibromoterephthalate. mp 126-127°C.
[0039] 1 H NMR (CDCl 3 , 300MHz, ppm) 8.22 (s, 2H), 4.40 (q, 4H, J = 7.1Hz), 1.40 (t, 6H, J = 7.20Hz).
Embodiment 2
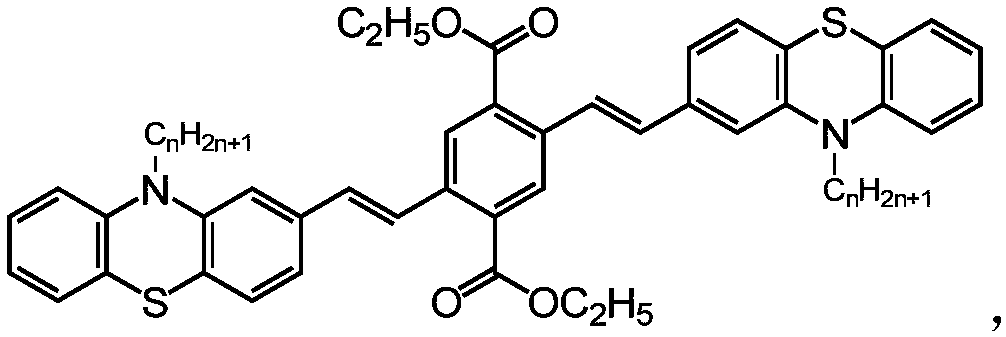
[0041] Get 10-ethyl-3-vinyl phenothiazine (0.50g), 2,5-dibromodiethyl terephthalate (0.40g), potassium carbonate (0.80g), tetrabutylammonium bromide ( 1.20 g) and 10 mg of palladium chloride were placed in a 50 mL round bottom flask, 20 mL of dry DMF was added, nitrogen gas flowed for 10 minutes, and the reaction was carried out under nitrogen protection at 120° C. for 12 hours. Cool, pour into 100mL water, filter with suction and dry. Purified by column chromatography (dichloromethane / petroleum ether (2:1) as eluent) to obtain a yellow solid: 2,5-bis((10-ethyl-10-hydrogen-phenothiazin-2-yl) Vinyl) diethyl terephthalate. Yield 87%.
[0042] 1 H NMR (400MHz, CDCl 3 ,ppm)8.17(d,J=1.9Hz,2H),7.75(d,J=16.2Hz,2H),7.29(d,J=6.9Hz,4H),7.17–7.08(m,4H),6.95( d,J=16.1Hz,2H),6.92–6.77(m,6H),4.44(qd,J=7.1,1.6Hz,4H),3.91(s,4H),1.50–1.35(m,12H). 13 C NMR (101MHz, CDCl 3,ppm)167.07,144.64,144.43,136.91,131.77,131.55,130.62,128.73,127.36,127.29,126.28,125.35,124.56,124.30,123.82,122.48,1...
Embodiment 3
[0044] Get 10-butyl-3-vinylphenothiazine (0.50g), 2,5-dibromodiethyl terephthalate (0.33g), potassium carbonate (0.75g), tetrabutylammonium bromide ( 1.10 g) and 10 mg of palladium chloride were placed in a 50 mL round-bottomed flask, 20 mL of dry DMF was added, nitrogen gas flowed for 10 minutes, and the reaction was carried out under nitrogen protection at 120° C. for 12 hours. Cool, pour into 100mL water, filter with suction and dry. Purified by column chromatography (dichloromethane / petroleum ether (2:1) as eluent) to obtain a yellow solid: 2,5-bis((10-butyl-10-hydrogen-phenothiazin-2-yl) Vinyl) diethyl terephthalate. Yield 89%.
[0045] 1 H NMR (400MHz, CDCl 3 ,ppm)8.18(s,2H),7.76(d,J=15.9Hz,2H),7.32(d,J=7.3Hz,4H),7.19–7.10(m,4H),6.97(d,J=16.1 Hz,2H),6.91–6.77(m,6H),4.44(q,J=7.2,4H),3.91(s,4H),1.86–1.74(m,4H),1.52–1.40(m,10H), 0.95(t,J=7.4Hz,6H). 13 C NMR (101MHz, CDCl 3 ,ppm)167.08,144.65,144.44,136.91,131.78,131.56,130.62,128.72,127.37,127.31,126.30,125.35,124.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com