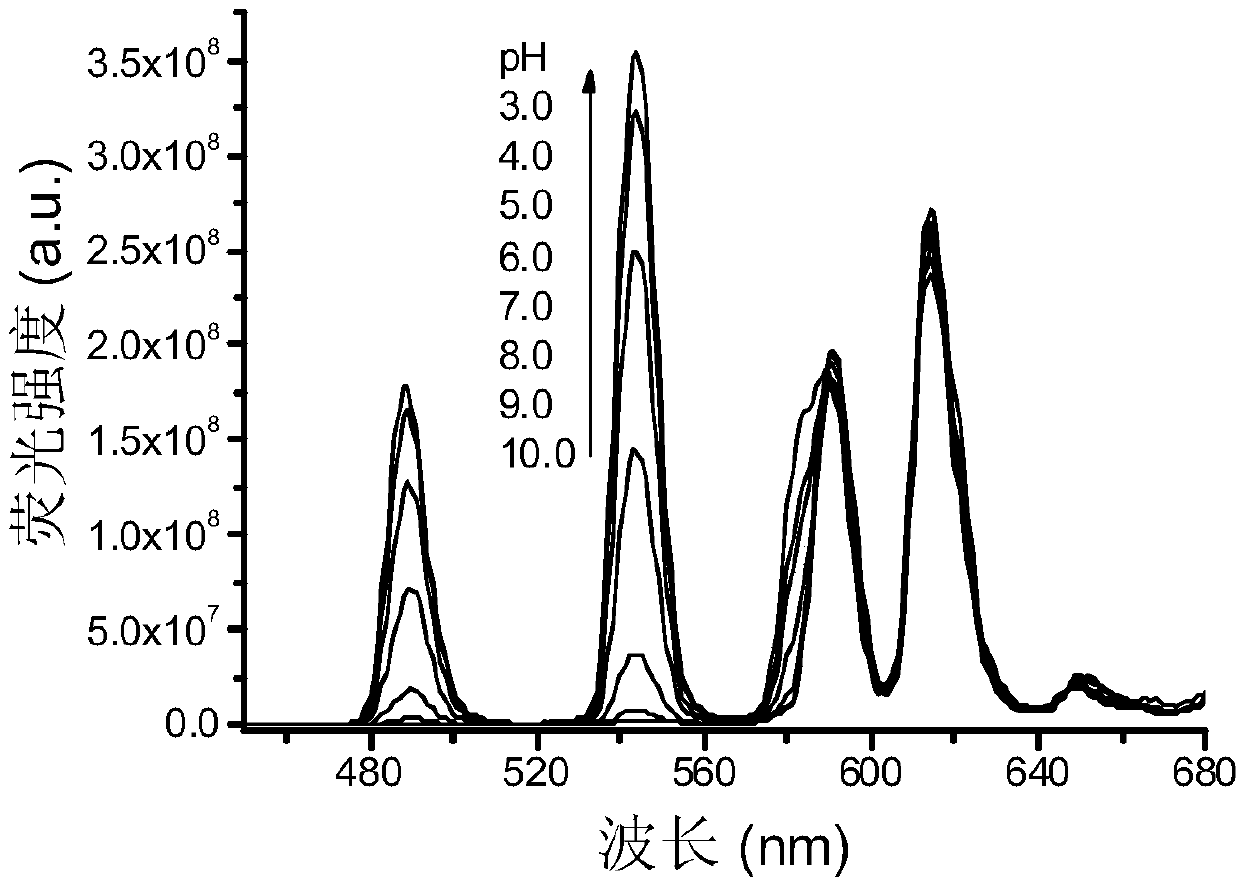
Rare-earth carbon nanoparticle, preparation method of rare-earth carbon nanoparticle and application for determining pH value based on fluorescence color scale
A carbon nanoparticle and nanoparticle technology, applied in the field of luminescence inspection, can solve the problems of complex organic chemical synthesis of molecular probes, large size of nanoparticle fluorescent probes, insoluble molecular probes in water, etc., and avoid complex chemical synthesis. , The effect of rapid pH response and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1 Preparation of Rare Earth Carbon Nanoparticles C:EuTbDPA
[0042]Add 0.5mmol europium nitrate, 0.5mmol terbium nitrate and 0.5mmol 2,6-pyridinedicarboxylic acid to 10mL polyethylene glycol 400 reagent in order to form polyethylene glycol 400: europium ion: terbium ion: 2,6-pyridine dicarboxylic acid The molar ratio of carboxylic acid is a mixed solution of 55:1:1:1; the reactant is stirred at 80°C for 10 minutes, then the temperature is raised to 100°C and stirred for 20 minutes, and finally stirred at 210°C for 6 minutes, and the heating equipment is terminated. The whole reaction needs to be carried out under nitrogen as protective gas. After cooling down to room temperature, add acetone solution and centrifuge to collect the pale yellow precipitate, then centrifuge and wash the precipitate twice with acetone and deionized water at a rotational angular speed of 14000 rad / s, and wash for 10 min each time, and finally dry the precipitate at 60°C Backup. f...
Embodiment 2
[0043] The preparation of embodiment 2 rare earth carbon nanoparticles C:EuTbDPA
[0044] Add 0.4mmol europium nitrate, 0.4mmol terbium nitrate and 0.5mmol 2,6-pyridinedicarboxylic acid to 10mL polyethylene glycol 400 reagent in order to form polyethylene glycol 400: europium ion: terbium ion: 2,6-pyridine dicarboxylic acid The molar ratio of carboxylic acid is a mixed solution of 55:0.8:0.8:1; the reactant is stirred at 80°C for 10 minutes, then the temperature is raised to 110°C and stirred for 20 minutes, and finally stirred at 210°C for 6 minutes to stop the heating equipment. The whole reaction needs to be carried out under nitrogen as protective gas. After cooling down to room temperature, add acetone solution and centrifuge to collect the pale yellow precipitate, then centrifuge and wash the precipitate twice with acetone and deionized water at a rotational angular speed of 14000 rad / s, and wash for 10 min each time, and finally dry the precipitate at 60°C Backup. The...
Embodiment 3
[0045] Preparation of Example 3 Rare Earth Carbon Nanoparticles C:EuTbDPA
[0046] Add 0.4mmol europium nitrate, 0.5mmol terbium nitrate and 0.5mmol 2,6-pyridine dicarboxylic acid to 10mL polyethylene glycol 400 reagent in order to form polyethylene glycol 400: europium ion: terbium ion: 2,6-pyridine dicarboxylic acid The molar ratio of carboxylic acid is a mixture of 55:0.8:1:1.1; the reactant is stirred at 80°C for 10 minutes, then the temperature is raised to 120°C and stirred for 30 minutes, and finally stirred at 230°C for 8 minutes, and the heating equipment is terminated. The whole reaction needs to be carried out under nitrogen as protective gas. After cooling down to room temperature, add acetone solution and centrifuge to collect the pale yellow precipitate, then centrifuge and wash the precipitate twice with acetone and deionized water at a rotational angular speed of 14000 rad / s, and wash for 10 min each time, and finally dry the precipitate at 60°C Backup. The a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com