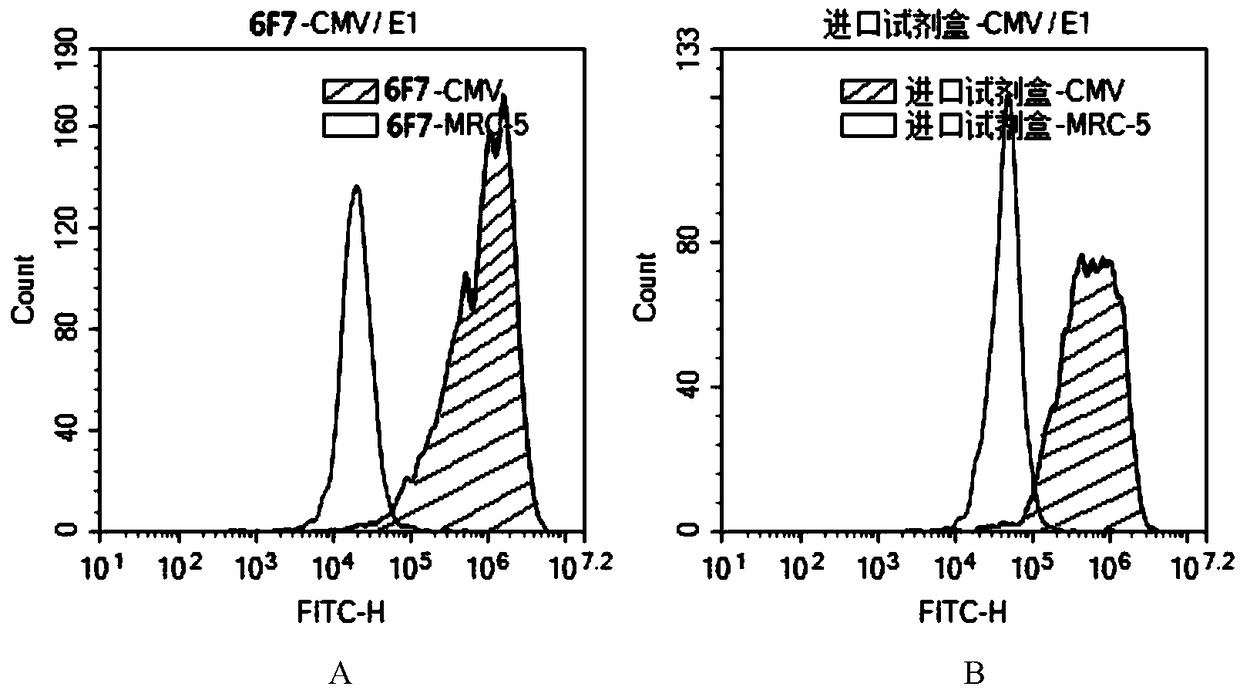
Hybridoma cell strain C11-6F7 as well as produced human cytomegalovirus (HCMV) monoclonal antibody and application thereof
A hybridoma cell line and monoclonal antibody technology, applied in biochemical equipment and methods, microbes, biological material analysis, etc., can solve the problems of low specificity, low sensitivity, and inability to solve active infection, etc., and achieve high purity , the effect of excellent performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Obtaining of HCMV-pp65-C214 monoclonal antibody
[0026] Prepare anti-HCMV-pp65-C214 monoclonal antibody according to the following steps:
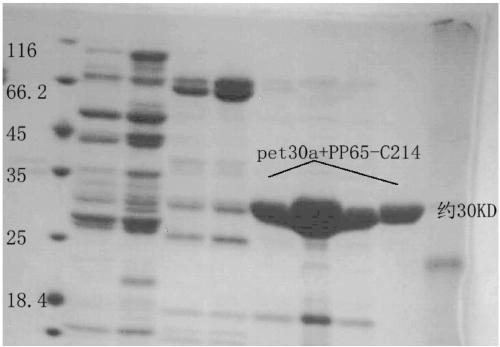
[0027] 1. Preparation of immunogen: The protein sequence encoded by UL83 was found from NCBI, and the 214 amino acids at the C-terminal were optimized according to the prokaryotic expression codons to synthesize the gene sequence, connected to the prokaryotic expression vector pet30a, and induced by IPTG. After the inclusion body was crushed by ultrasonic waves, the precipitate was dissolved in 8M urea and then purified by a nickel column. The miscellaneous proteins were washed with 20mM imidazole, and the proteins eluted with 50mM, 100mM, and 200mM imidazole were collected respectively. The eluted products were identified by SDS-PAGE. The electrophoresis results are shown in the figure As shown in 1, the one marked PP65-C214 in the figure is the target protein eluted from 200mM imidazole, and the electrophoresis picture...
Embodiment 2
[0030] Embodiment 2: Ascites titer and sensitivity measured by indirect ELISA
[0031] 2.1 Ascites titer: The antigen PP65-C214 obtained in Example 1 was diluted with carbonate buffer solution and coated on a 96-well plate, 100ng / well, incubated at 37°C for 2 hours, and then PBS -T Wash the plate once. Add PP65-C214 ascites with different dilutions obtained in Example 1 and incubate at 37°C for 30 minutes, set up negative control wells, positive control wells (3E4) and blank control wells (antibody dilution), and wash the plate with PBS-T after incubation for 5 minutes Second-rate. Dilute the HRP-labeled antibody (goat anti-mouse IgG-HRP) by 1000 times with enzyme label diluent, 100 μl per well. Incubate at 37°C for 30min. After incubation, the plate was washed 5 times with PBS-T. Color development, liquid A: liquid B = 1:1, ready to use. 100 μl per well, incubate at 37°C for 15 minutes; add stop solution, 50 μl / well, to terminate the color reaction, and detect the absorb...
Embodiment 3
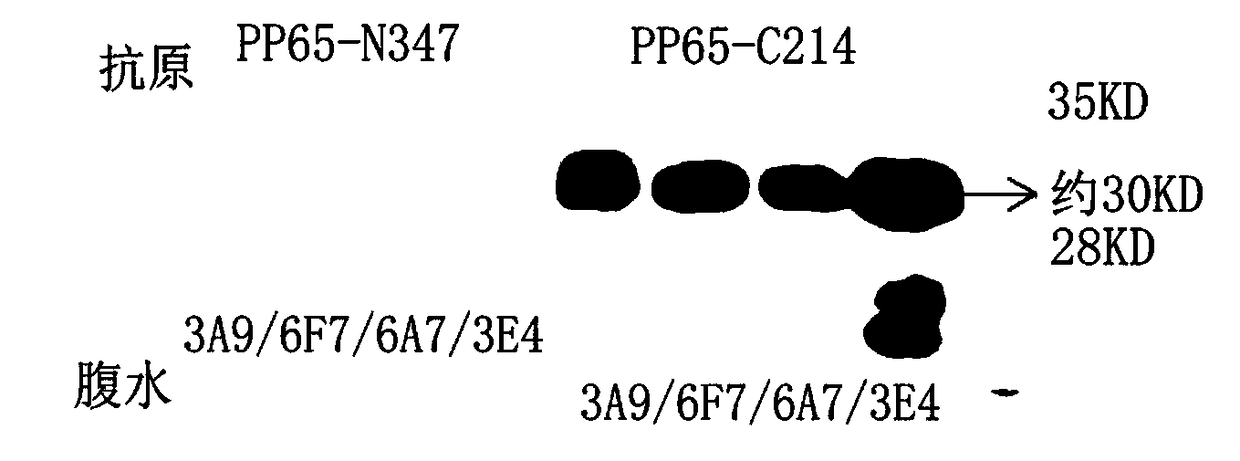
[0037] Example 3 Specific detection of ascites and further verification by Western Blot
[0038] 3.1 Specificity detection: PP65-N347 antigen and PP65-C214 antigen encoded by the N-terminal 1041bp of pp65 gene were respectively coated, and the cross-reaction of 6F7 antibody to PP65-N347 was detected by competitive ELISA method to detect antibody specificity. Coat the 96-well plate after diluting with salt coating solution, 100 / 50ng per well, incubate at 37°C for 2 hours, and wash the plate once with PBS-T after the incubation. Add monoclonal antibody, incubate at 37°C for 30min, blank control (antibody diluent). After incubation, the plate was washed 5 times with PBS-T. Dilute the HRP-labeled antibody (goat anti-mouse IgG-HRP) 1000 times with 100 μl per well. Incubate at 37°C for 30min. After incubation, the plate was washed 5 times with PBS-T. Chromogenic solution A: Chromogenic solution B = 1:1, ready to use. 100 μl per well, incubated at 37°C for 15 minutes; adding sto...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com