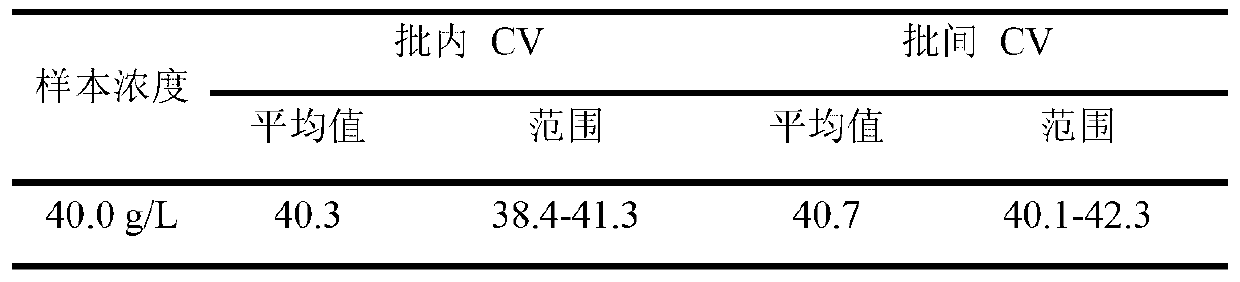
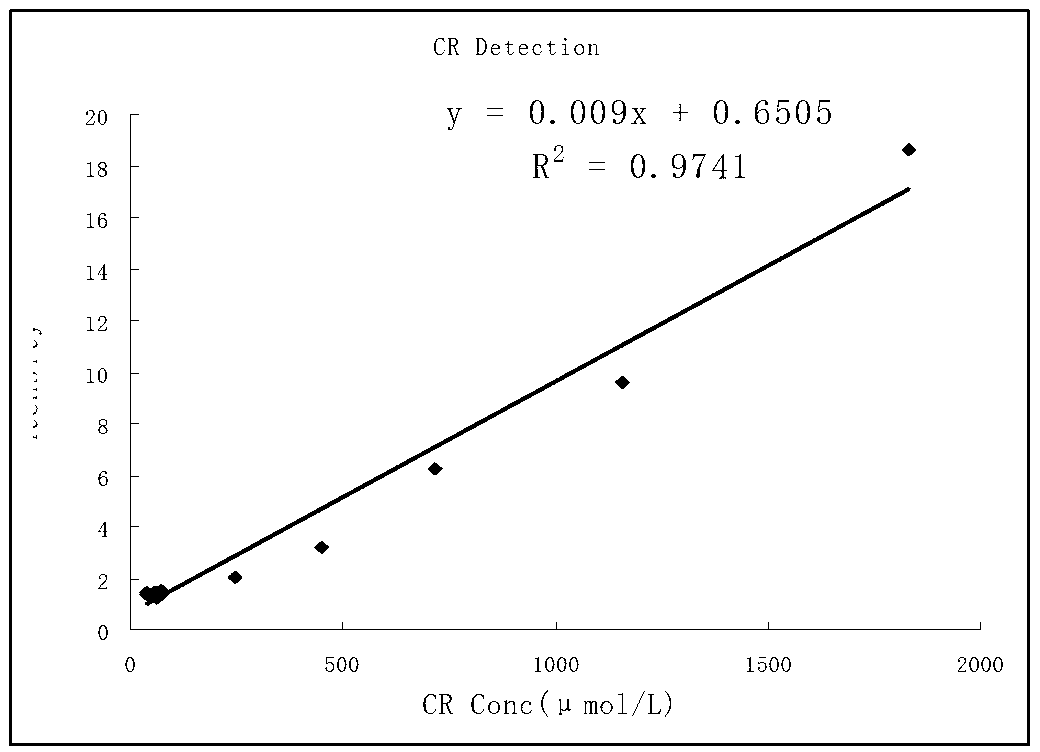
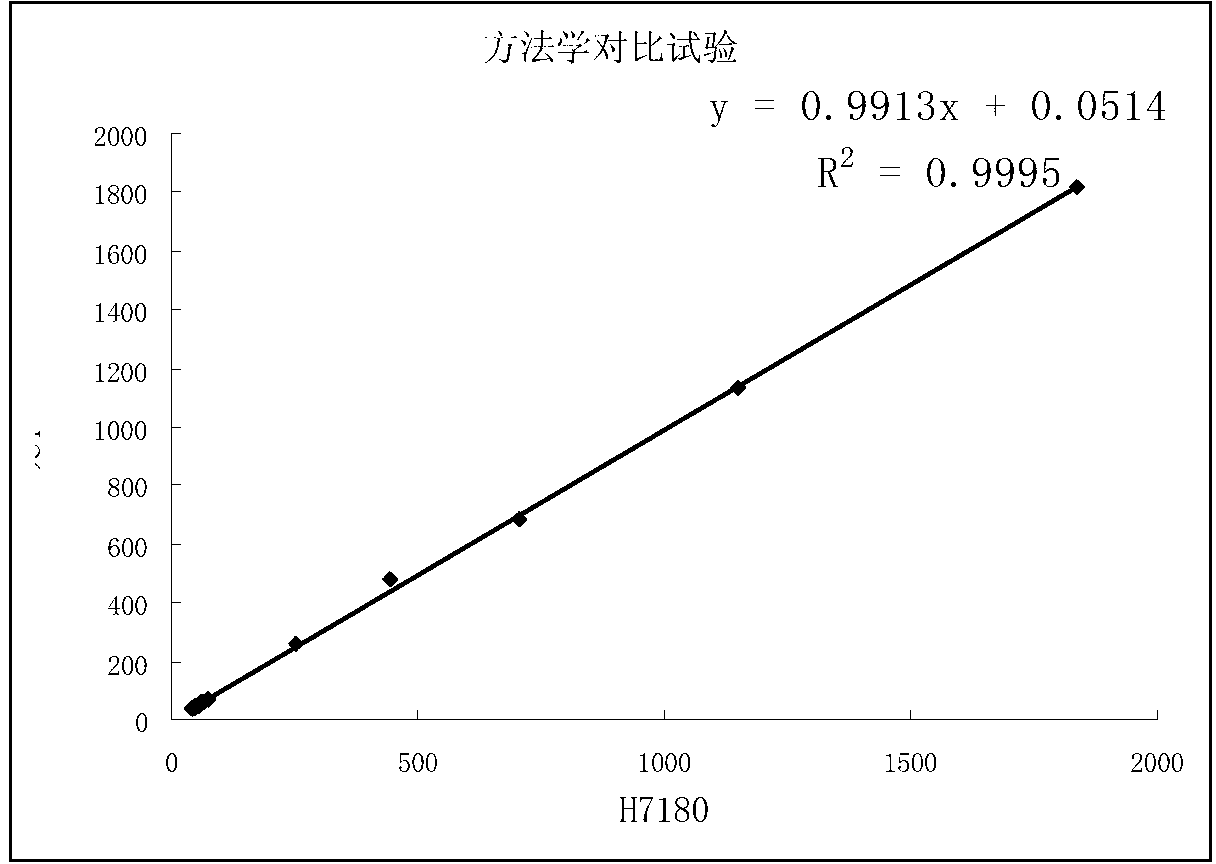
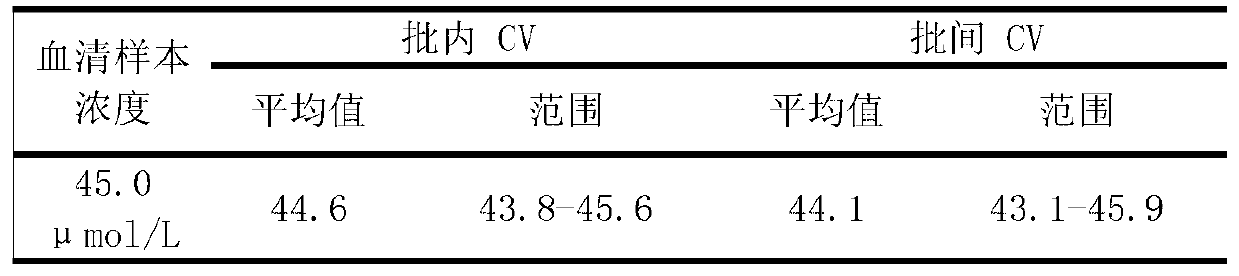
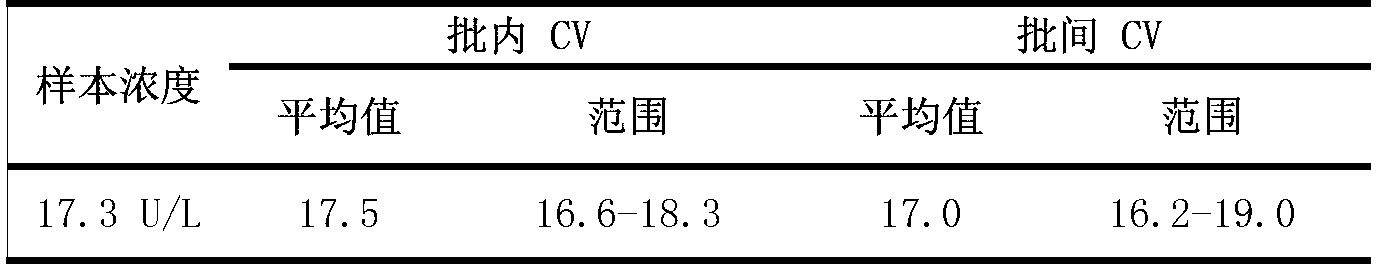
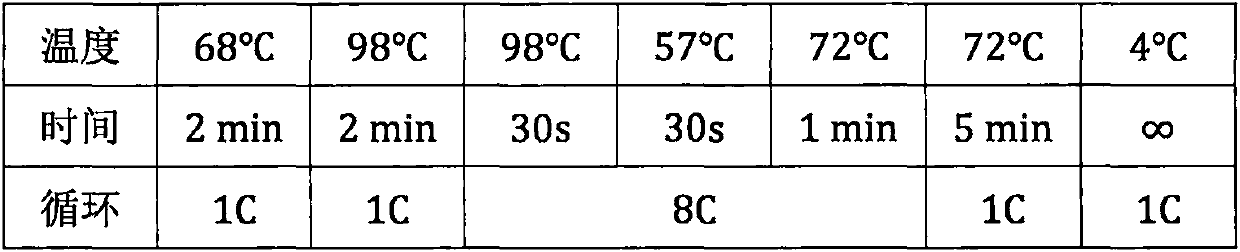
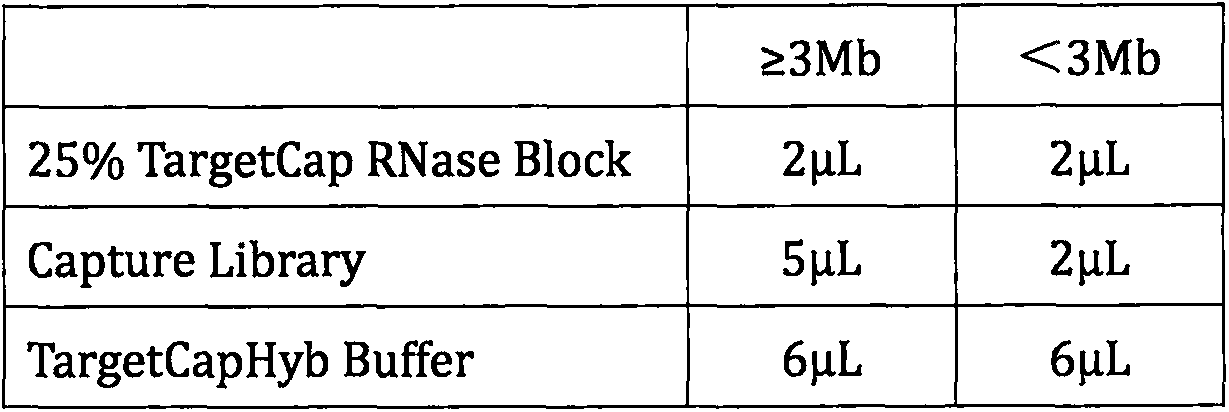
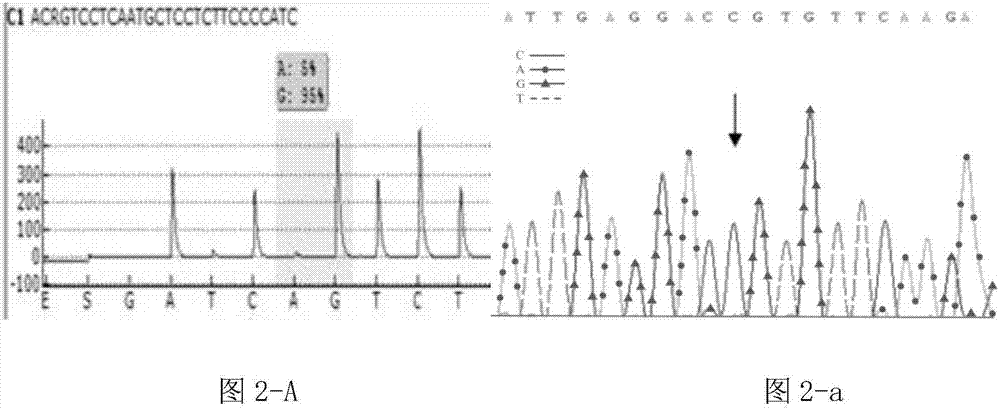
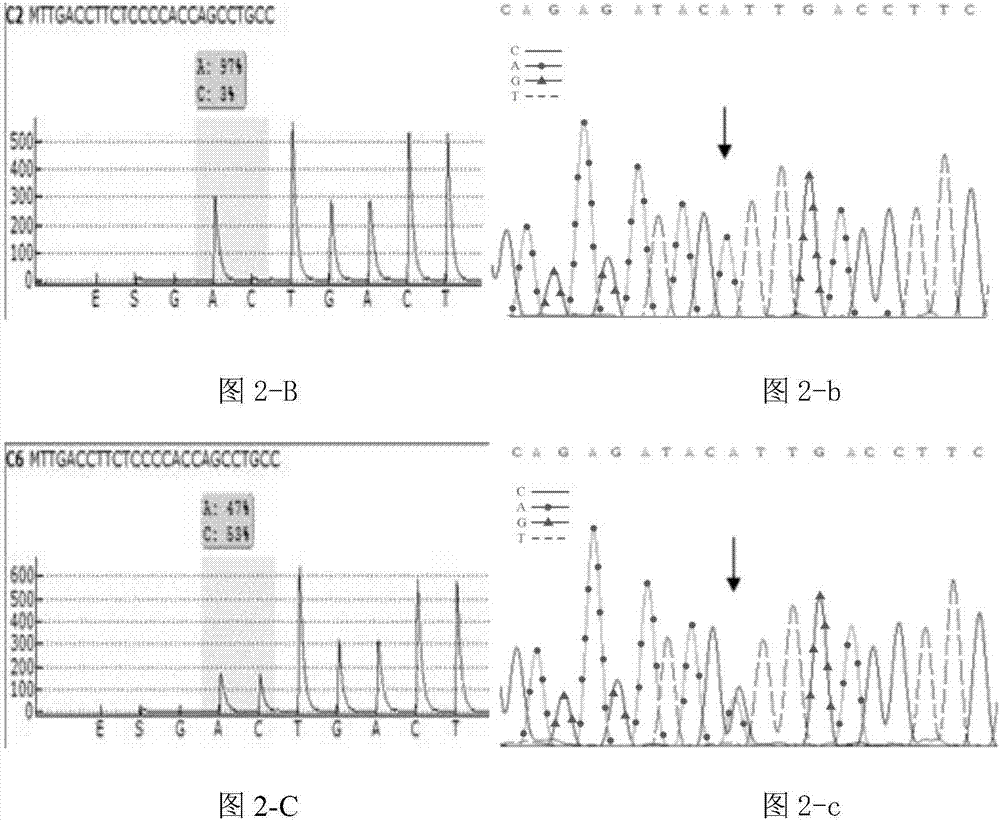
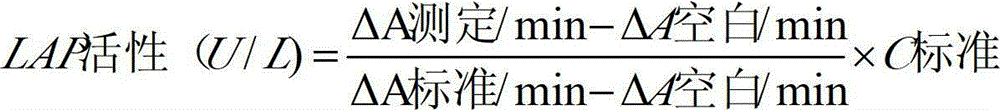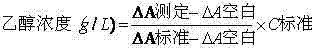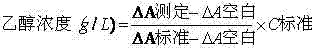Patents
Literature
66results about How to "Meet clinical testing requirements" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Kit for testing and identifying genetic cardiac hypertrophy related gene mutation
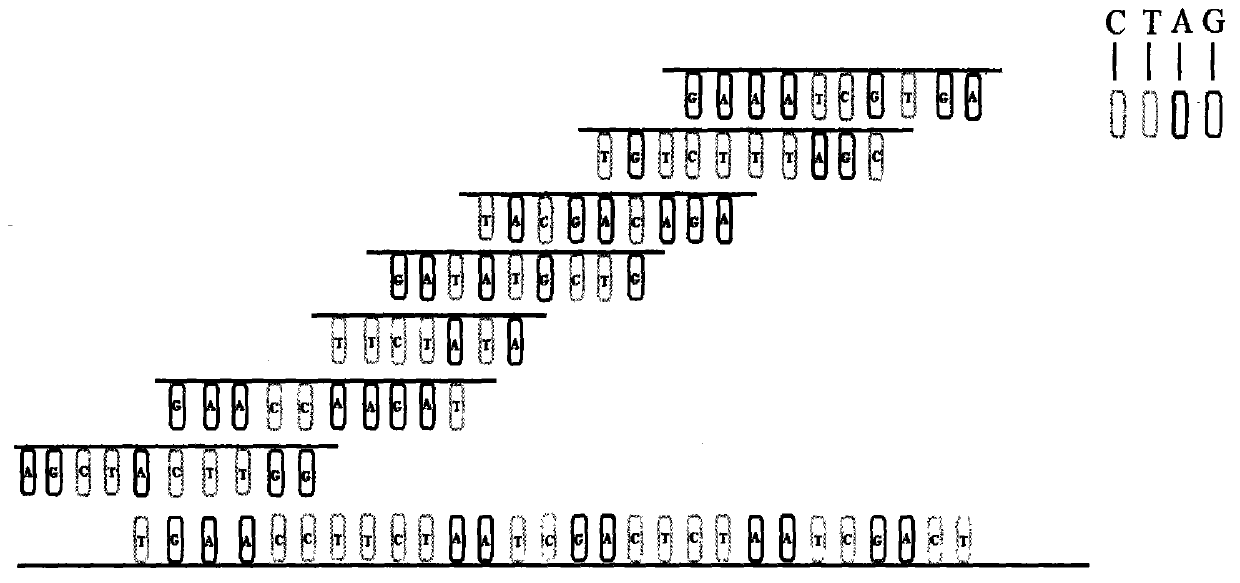
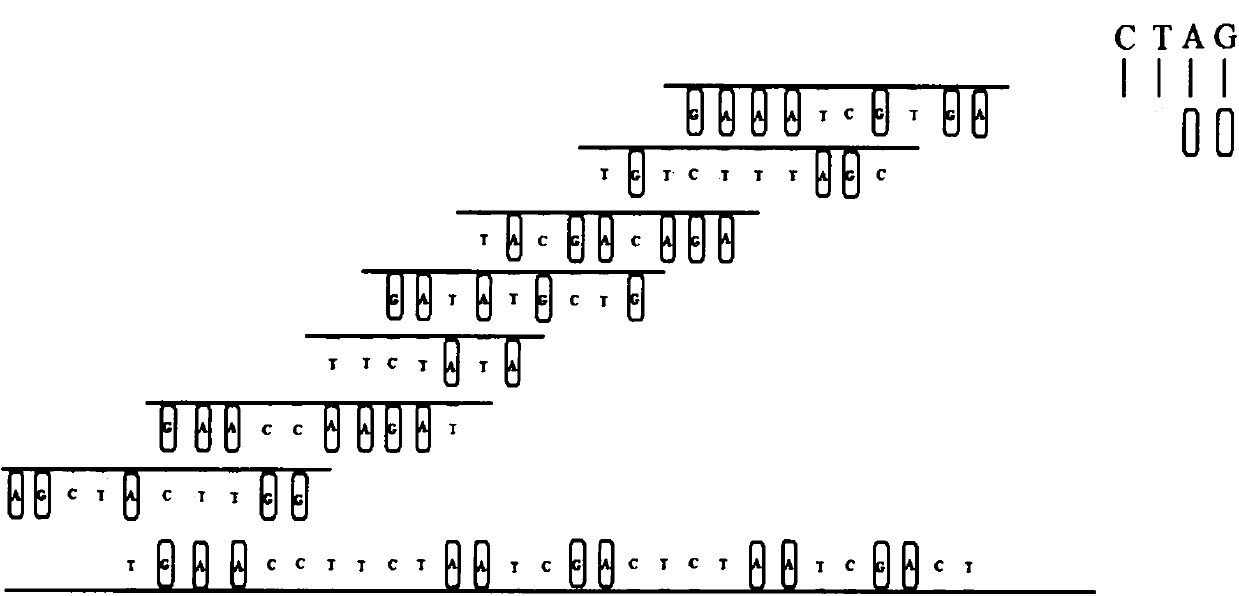
The invention relates to a kit for detecting genetic cardiac hypertrophy related gene mutation samples, particularly to a product for detecting genetic cardiac hypertrophy related genes by a massively parallel sequencing platform technology. The method involved in the kit comprises: a) uniquely designing and preparing a capture probe, which is directed at all exon fragments of genes ACTC1, ACTN2, BRAF, CALR3, CASQ2, CSRP3, GLA, HRAS, JPH2, KRAS, LAMP2, LDB3, MAP2K1, MYBPC3, MYH6, MYH7, MYL2, MYL3, MYLK2, MYOZ2, PRKAG2, RAF1, SOS1, TCAP, TNNC1, TNNI3, TNNT2, TPM1, TTN, TTR, and VCL; b) designing unique joints with tag sequences; c) using universal primers to conduct PCR amplification on probe sequences; and d) designing unique operations for capture of mixed target fragments. The massively parallel sequencing platform prepared by the method and the kit has the advantages of high sample throughput, high efficiency, and easy operation, thus greatly reducing the sequencing testing cost.
Owner:康旭基因技术(北京)有限公司
Albumin detection reagent
The invention discloses an albumin detection reagent which comprises a diluent and a reaction reagent, wherein the diluent is composed of trihydroxymethyl amino buffer, surfactant, preservative, anti-bilirubin interference agent and vitamin C oxidase; and the reaction reagent is composed of buffer, surfactant, bromocresol green and freeze-drying protective agent. The detection reagent disclosed by the invention has favorable sensitivity, accuracy, precision and linearity, and can completely satisfy the clinical examination requirements.
Owner:宁波美康盛德医学检验所有限公司
Creatinine detection reagent
ActiveCN103278468AHigh sensitivityImprove accuracyColor/spectral properties measurementsMethylanilineCreatininase
The invention discloses a creatinine detection reagent which is characterized by comprising a diluent and a reaction reagent, wherein the diluent is composed of buffer, surfactant, preservative, anti-bilirubin interference agent and vitamin C oxidase; and the reaction reagent is composed of buffer, creatinase, sarcosine oxidase, peroxidase, N-ethyl-N-(3-propylsulfo)-3-methylaniline, creatininase, 4-aminoantipyrine, preservative and freeze-drying protective agent. The detection reagent disclosed by the invention has favorable sensitivity, accuracy, precision and linearity, and can completely satisfy the clinical examination requirements.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Kit for detecting neurofibromatosis 1 (NF1)-related gene mutation
InactiveCN104450885AImprove capture efficiencyLow costMicrobiological testing/measurementPolymerase chain reactionNeurofibromatosis
The invention relates to a sample preparation kit for detecting neurofibromatosis 1 (NF1)-related gene mutation, and in particular relates to a product for detecting NF1-related gene by the massively parallel sequencing platform technology. The kit is that A, a capture probe is specially designed and prepared for all exon fragments of NF1 gene; B, an unique linker with a tag sequence is designed; C, RCP (Polymerase Chain Reaction) amplification is performed for the probe sequence through a primer; and D, the operation of capturing a targeted fragment after mixing is specially designed. A massively parallel sequencing platform prepared through the method and the kit is high in sample throughput, high in efficiency, and simple and convenient to operate, and the sequencing inspection cost is greatly decreased.
Owner:百世诺(北京)医疗科技有限公司
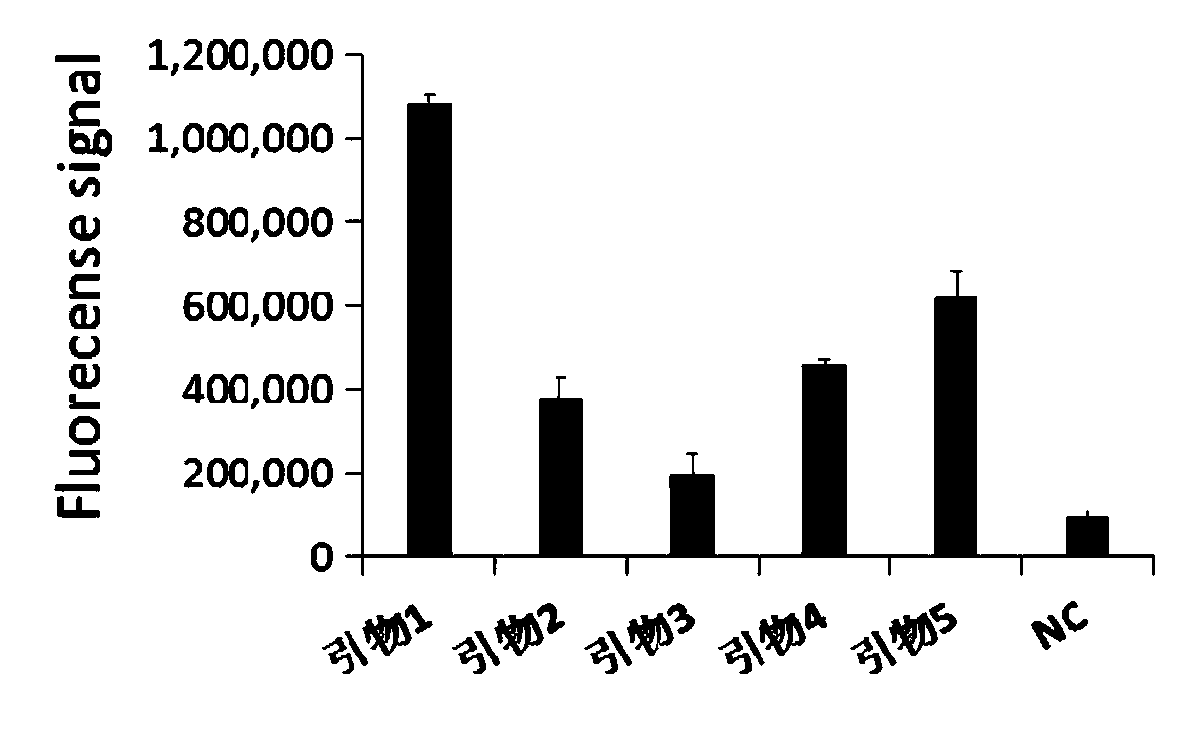
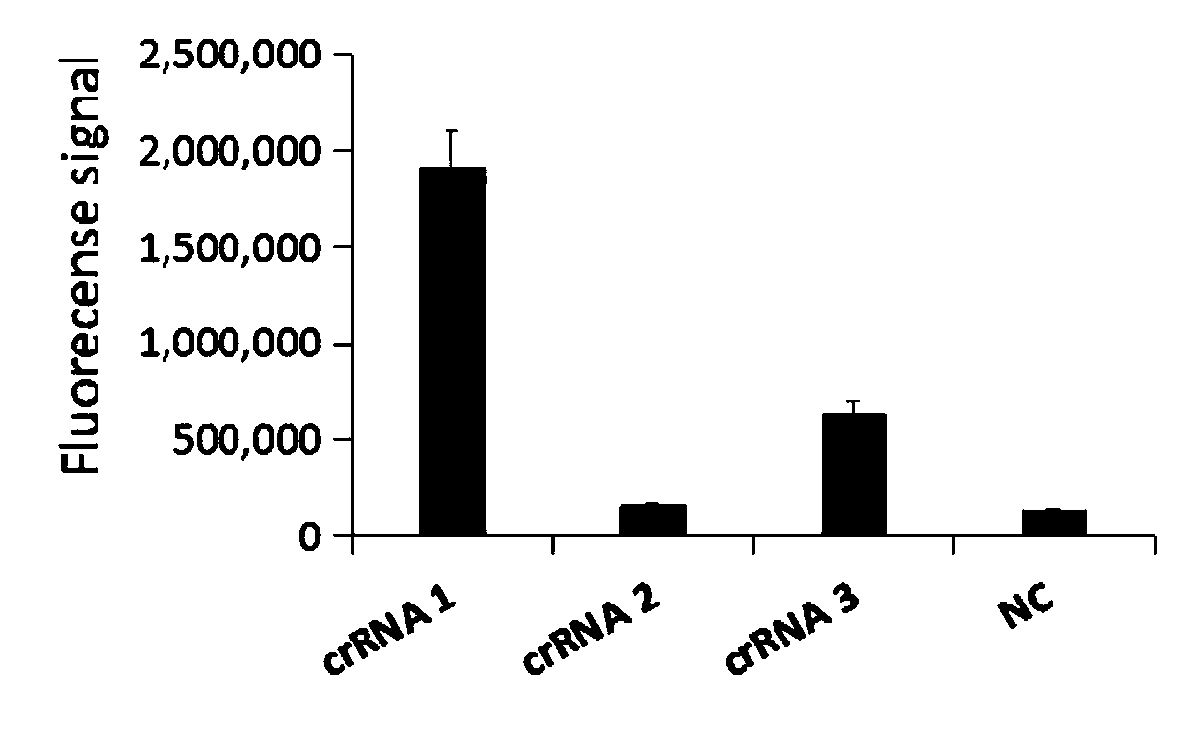
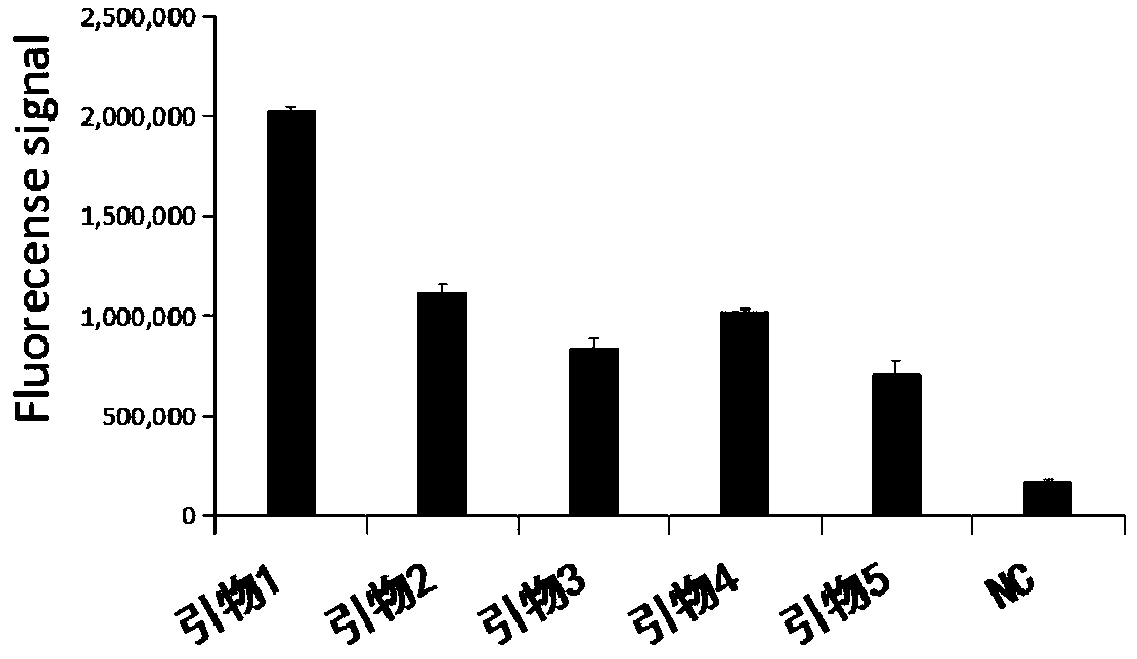
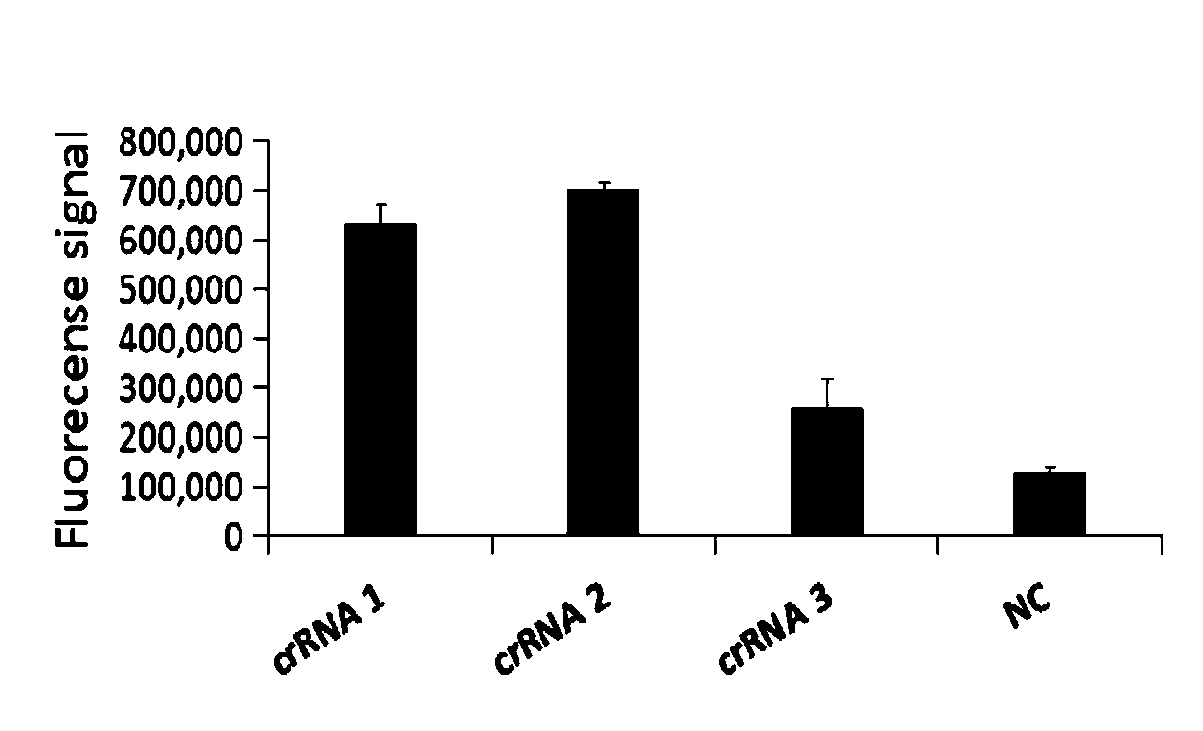
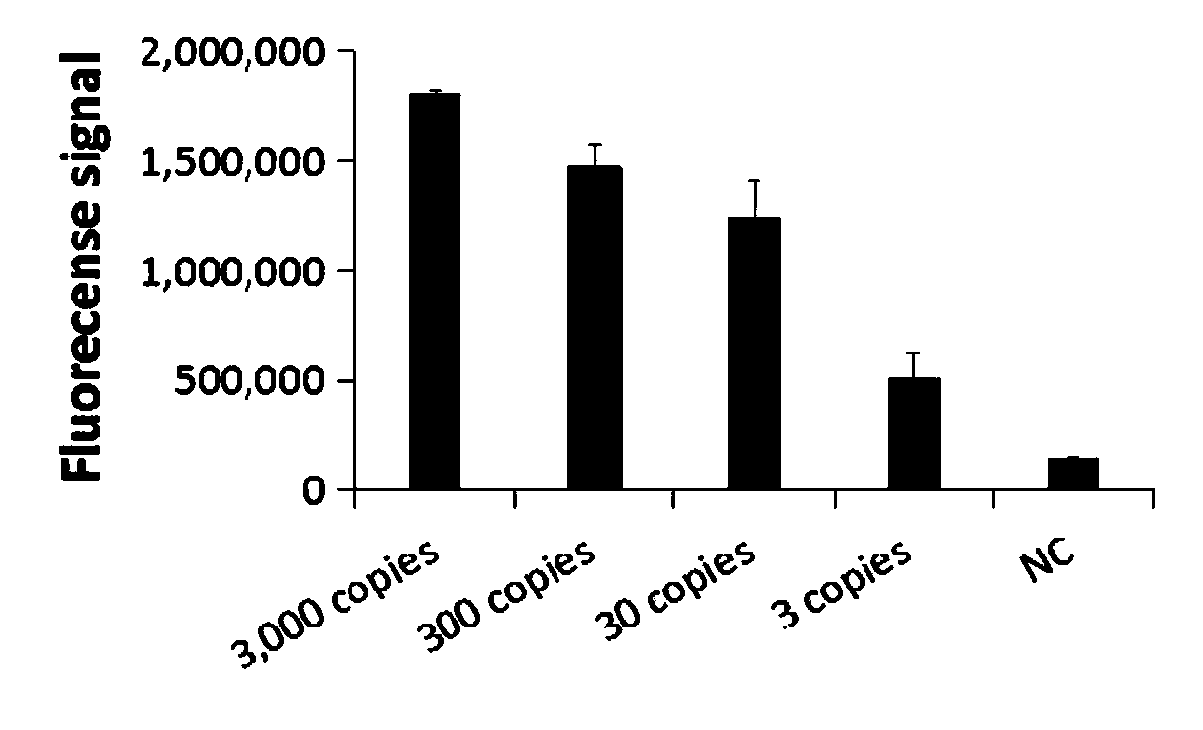
Primer group for rapid detection of 2019-nCoV based on CRISPR technology and application thereof
ActiveCN111363847ABroad application prospectsShorten detection timeMicrobiological testing/measurementMicroorganism based processesMolecular biologyRapid detection
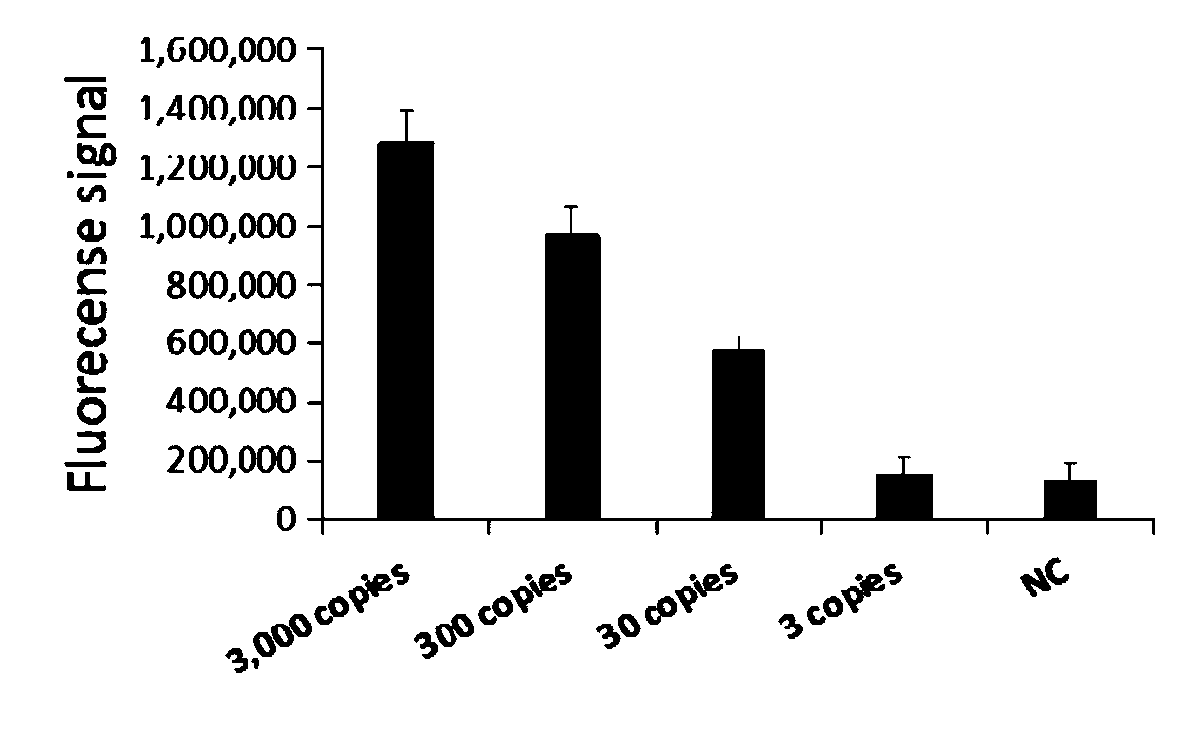
The invention relates to a primer group for rapid detection of 2019-nCoV based on a CRISPR technology and application thereof, and belongs to the technical field of gene detection of the CRISPR technology. The primer group comprises an Orf1ab gene amplification primer pair, an Orf1ab gene crRNA, an N gene amplification primer pair and an N gene crRNA. The primer group is adopted to detect 2019-nCoV by the CRISPR technology, the detection time of 2019-nCoV is shortened, and detection can be completed within 40-60min. A specific sequence combination is obtained through screening to serve as theprimer group for detection, the primer group has the advantages of being high in sensitivity and specificity, and the detection limit can reach 7.5copies. The primer group is adopted to conduct CRISPRdetection on 2019-nCoV, dependence on complex variable-temperature amplification instruments such as a qPCR instrument is eliminated, and the CRISPR-Cas technology has wide application prospects in the aspect of real-time diagnosis of 2019-nCoV.
Owner:广州微远医疗器械有限公司 +4
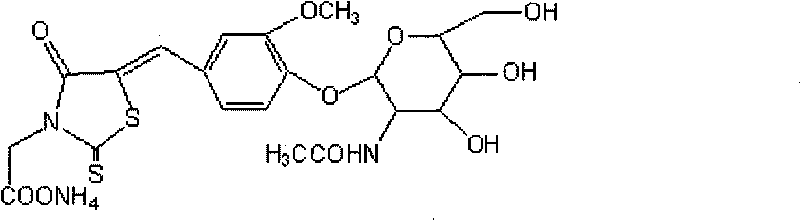
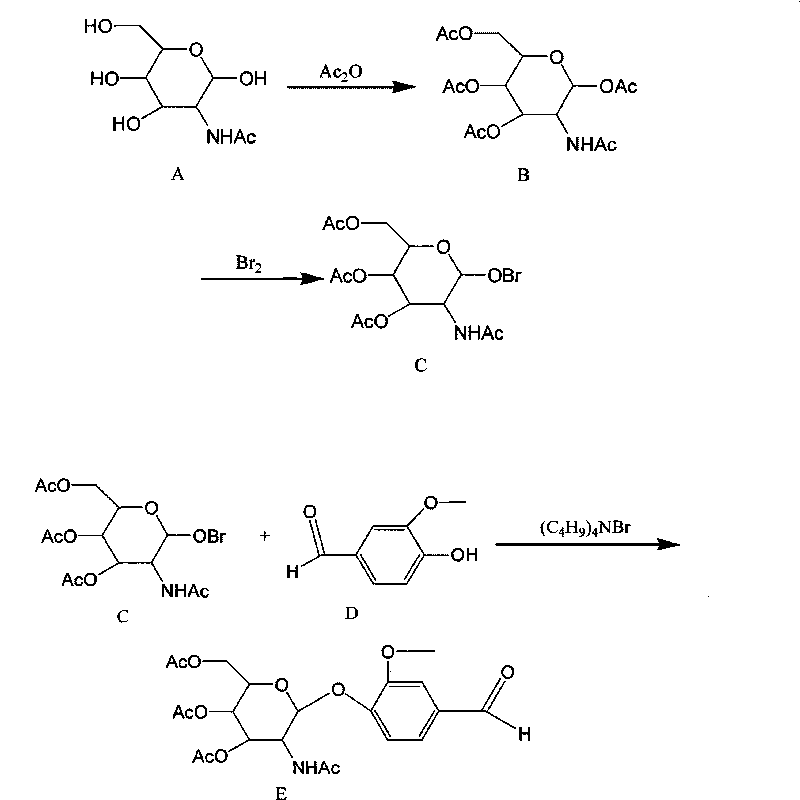
Liquid reagent for determining N-acetyl-beta-D-glucosaminidase
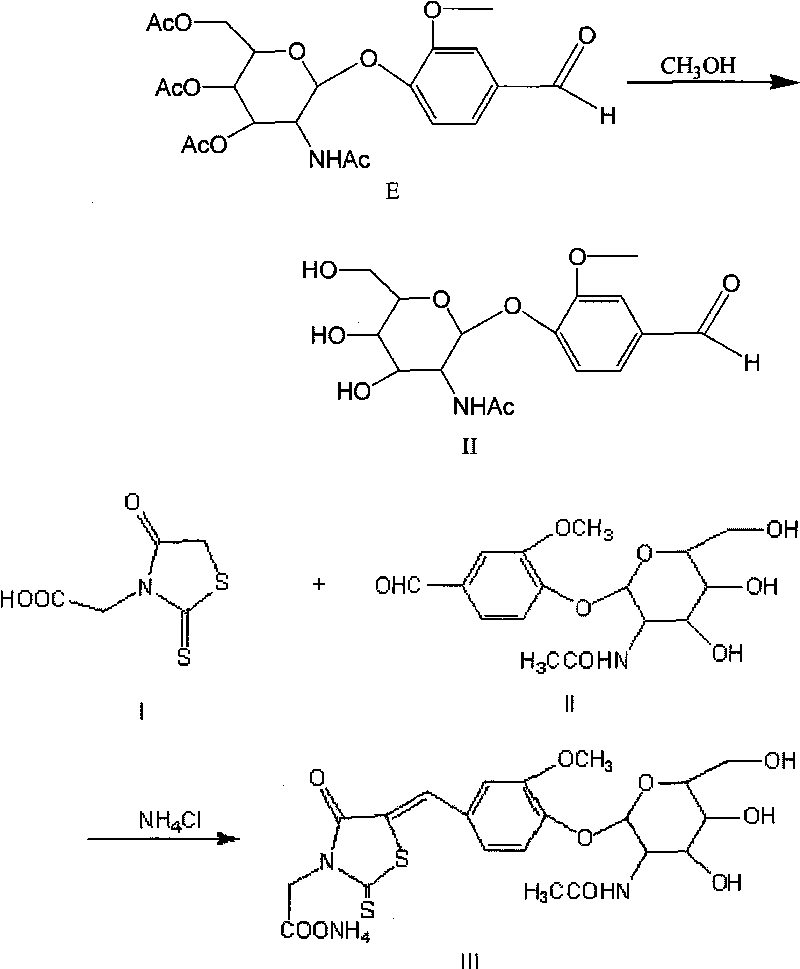
ActiveCN101738379AReduce distractionsMeet clinical testing requirementsSugar derivativesColor/spectral properties measurementsSolubilityRhodanine
The invention discloses a liquid reagent for determining N-acetyl-beta-D-glucosaminidase, which takes 5-(4-(3-methoxyl-phenylmethene-)-rhodanine-3-qmmonium acetate-N-acetylamino-beta-D-glucoside as substrate and is prepared by adding proper buffer solution, surfactant, preservative, stabilizer and the like. The liquid reagent has the advantages of large solubility of the substrate, strong stability, high detection sensitivity, convenient clinical detection, low detection cost and small outside interference for the detection result of N-acetyl-beta-D-glucosaminidase.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Detection reagent for alanine aminotransferase
ActiveCN103320497AHigh sensitivityImprove accuracyMicrobiological testing/measurementThiamine pyrophosphatePhosphoric acid
The invention discloses a detection reagent for alanine aminotransferase. The detection reagent comprises a diluent and a reaction reagent. The diluent comprises a buffer, a surfactant, an antiseptic, a bilirubin-interference removing reagent, and an ascorbic oxidase. The reaction reagent comprises a buffer, L-alanine, alpha-ketoglutaric acid, phosphoric acid, pyruvate oxidase, thiamine pyrophosphate, magnesium chloride, peroxidase, 4-aminoantipyrene, a chromogen, an antiseptic, and a freeze-drying protecting agent. The detection reagent has advantages of good sensitivity, accuracy, precision and linearity, and can completely meet the requirements of clinical examination.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
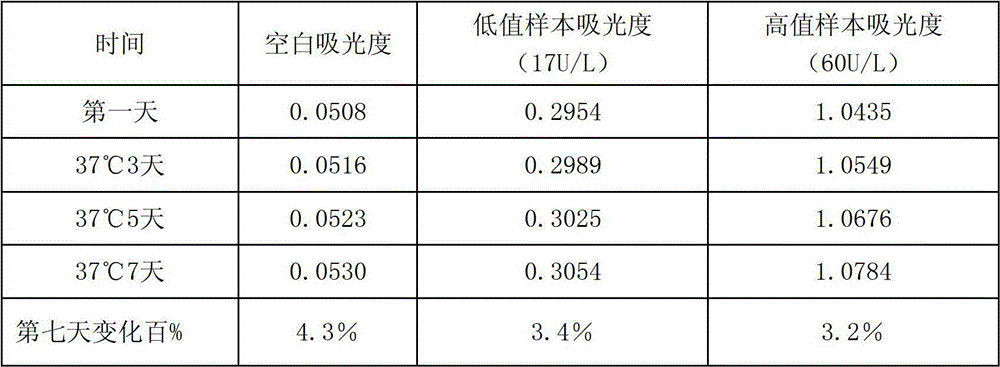
Anti-heparin interference leucine aminopeptidase measuring reagent
InactiveCN102864206AGood anti-heparin interference performanceImprove stabilityMicrobiological testing/measurementHydrogenPreservative
The invention discloses an anti-heparin interference leucine aminopeptidase measuring reagent, which consists of a reagent 1 and a reagent 2, wherein the reagent 1 and the reagent 2 comprises the following components in the concentration ranges: the reagent 1 comprises 50-500 mmol / L of buffer solution (pH (Potential of Hydrogen) of 3.0-6.0), 1-100 ml / L of anti-interference agent and 0.1-100 g / L of preservative; and the reagent 2 comprises 50-500 mmol / L of buffer solution (pH (Potential of Hydrogen) of 3.0-6.0), 1-100 ml / L of L-leucine-p-nitroxyl aniline, 1-100 g / L of stabilizer and 0.1-100 g / L of preservative. The anti-heparin interference leucine aminopeptidase measuring reagent has the advantages of good anti-heparin interference capacity, good stability and capability of completely meeting requirements of clinical inspection.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
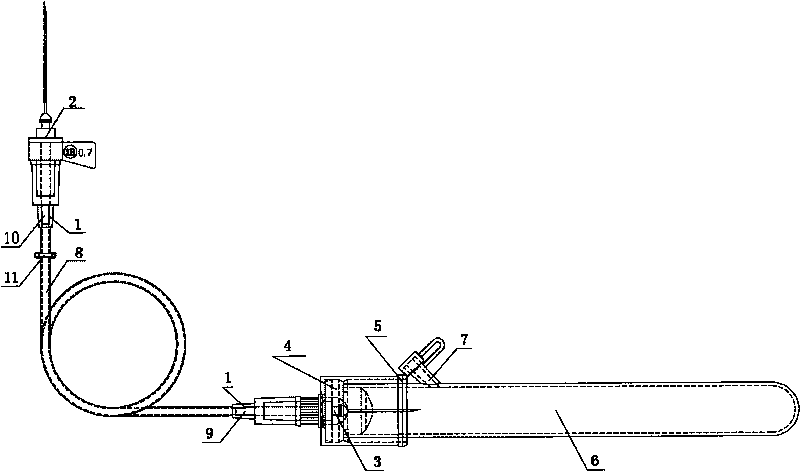
Disposable soft connecting band collection container and anti-freezing vacuum blood taking needle of bleeding opening
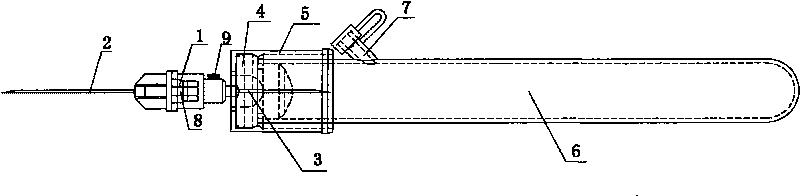
InactiveCN101695446AThe test results are accurateImprove the level of clinical treatmentCatheterDiagnostic recording/measuringCavity wallBiomedical engineering
The invention belongs to the technical field of medical instruments, and relates to a disposable soft connecting band collection container and an anti-freezing vacuum blood taking needle of a bleeding opening. The disposable soft connecting band collection container is formed in the following mode that: a biological product anticoagulant coating layer is arranged on an inner cavity wall of a circular connector; one end of the connector is provided with a blood taking plastic duct; a vacuum valve is placed on the plastic duct; the other end of the plastic duct is provided with the circular connector; the inner cavity wall of the circular connector is provided with the biological product anticoagulant coating layer; one end of the connector is provided with a venous blood taking needle, while the other end is provided with a blood collection sample bottle needle; the blood collection sample bottle needle is connected with a blood collection sample bottle cap; the blood collection sample bottle cap is connected with a rubber plug; the rubber plug is connected with a blood collection sample bottle; and one side on the blood collection sample bottle close to the blood collection sample bottle cap is provided with a secondary bleeding opening. The disposable soft connecting band collection container and the anti-freezing vacuum blood taking needle of the bleeding opening can ensure that a blood sample generates no cruor and hemolysis so as to ensure the accuracy of the test result. The blood dosage during the test can be controlled flexibly so as to ensure repeated tests at any time or the addition of new testing items.
Owner:天津百新生物技术研发有限公司
Determination reagent of total bile acid
The invention relates to a determination reagent of a total bile acid. The reagent comprises two components, wherein the reagent 1 comprises a phosphate buffer, Thio-NAD (Nicotinamide Adenine Dinucleotide), Emulgen B-66 CTAB (Cetyltrimethyl Ammonium Bromide), NaCl, 2-hydroxypyridine-N-oxide; the reagent 2 comprises a glycinate buffer, dextran sulfate, EGTA (Ethylene Glycol Tetraacetic Acid), 3 alpha-HSD (Hydrogen Sterol Dehydrogenase), Emulgen B-66, NADH (Nicotinamide-adenine Dinucleotide Acid), and 2-hydroxypyridine-N-oxide. As the combination of the cation surfactant and the nonionic surfactant Emulgen B-66 is added, the interference of bilirubin can be effectively eliminated; the selected stabilizer is added in the product, so that the reagent can be stored for one year at 2-8 DEG C without influence on the properties; the substrate concentration and the ion strength can be adjusted to enable the recovery rate of the reagent to be more than 98%. The determination reagent disclosed by the invention has the advantages of high accuracy in determined results, strong anti-interference performance, good stability and the like.
Owner:武汉利弘生物科技有限责任公司
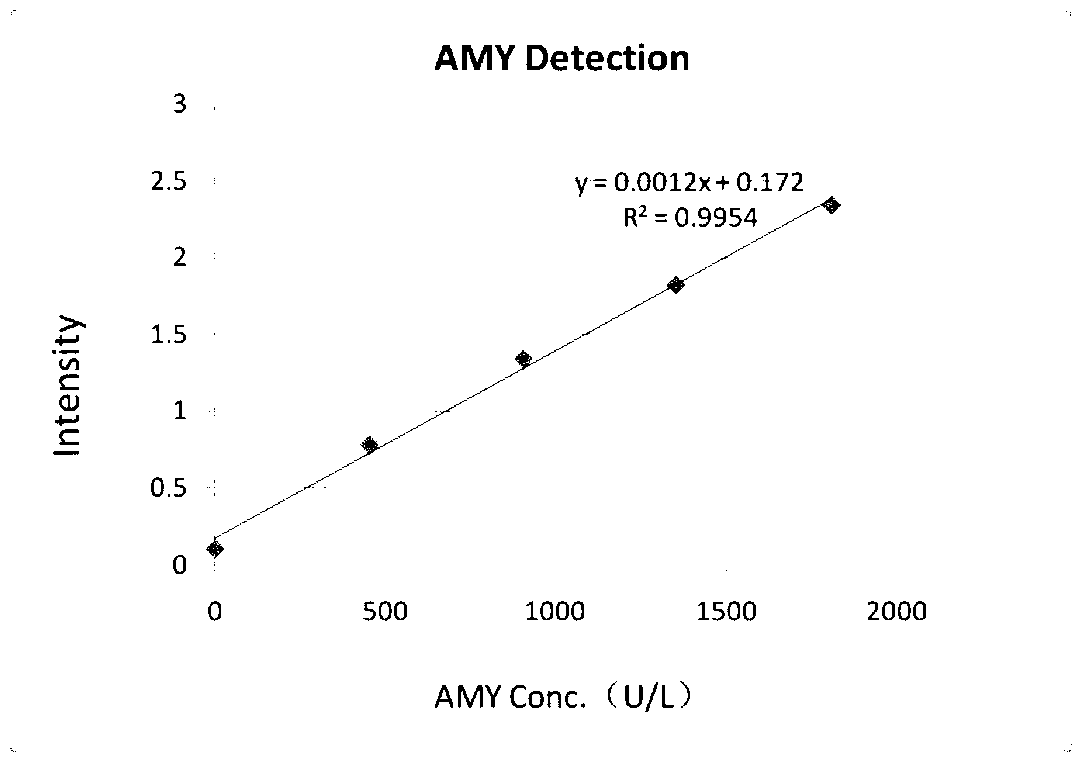
Amylase detection reagent
ActiveCN103266165AHigh sensitivityImprove accuracyMicrobiological testing/measurementVitamin CFreeze-drying
The invention discloses an amylase detection reagent which comprises a diluent and a reaction reagent, wherein the diluent is composed of a buffer solution, a surfactant, a preservative, a bilirubin interference remover and a vitamin C oxidase; and the reaction reagent is composed of a buffer solution, a substrate, an alpha-glucosaccharase, sodium chloride, calcium chloride, a preservative and a freeze-drying stability protective agent. The detection reagent disclosed by the invention has favorable sensitivity, accuracy, precision and linearity, and can completely satisfy the clinical examination requirements.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
CRISPR detection primer group for mycoplasma pneumoniae and application of CRISPR detection primer group
ActiveCN110804669AGet rid of dependenceShorten detection timeMicrobiological testing/measurementMicroorganism based processesMycoplasmaSequenceome
The invention relates to a CRISPR (clustered regularly interspaced short palindromic repeat) detection primer group for mycoplasma pneumoniae and an application of the CRISPR detection primer group, and belongs to the technical field of gene detection of a CRISPR technology. The primer group comprises an amplification primer pair and crRNA (CRISPR-derived ribonucleic acid), wherein the amplification primer pair is used for amplifying a sequence shown as SEQ ID NO. (sequence identifier number) 1 of the mycoplasma pneumoniae; crRNA comprises an anchor sequence and a guide sequence; the anchor sequence specifically identifies a Cas protein; and the guide sequence is matched with a target sequence segment in the sequence of SEQ ID NO. 1. The primer group detects the mycoplasma pneumoniae through the CRISPR technology; the detection time of the mycoplasma pneumoniae is shortened; and the detection can be accomplished within 60min. A specific sequence combination obtained by screening acts as the primer group for detection, the primer group has the advantages of high sensitivity and strong specificity, and a limit of detection of the primer group can reach 30 copies. The primer group isused for the CRISPR detection of the mycoplasma pneumoniae, is independent of a complicated variable temperature amplification instrument such as a qPCR (quantitative polymerase chain reaction) instrument, and a CRISPR-Cas technology has wide application prospects in instant diagnosis of the mycoplasma pneumoniae.
Owner:广州微远医疗器械有限公司 +3
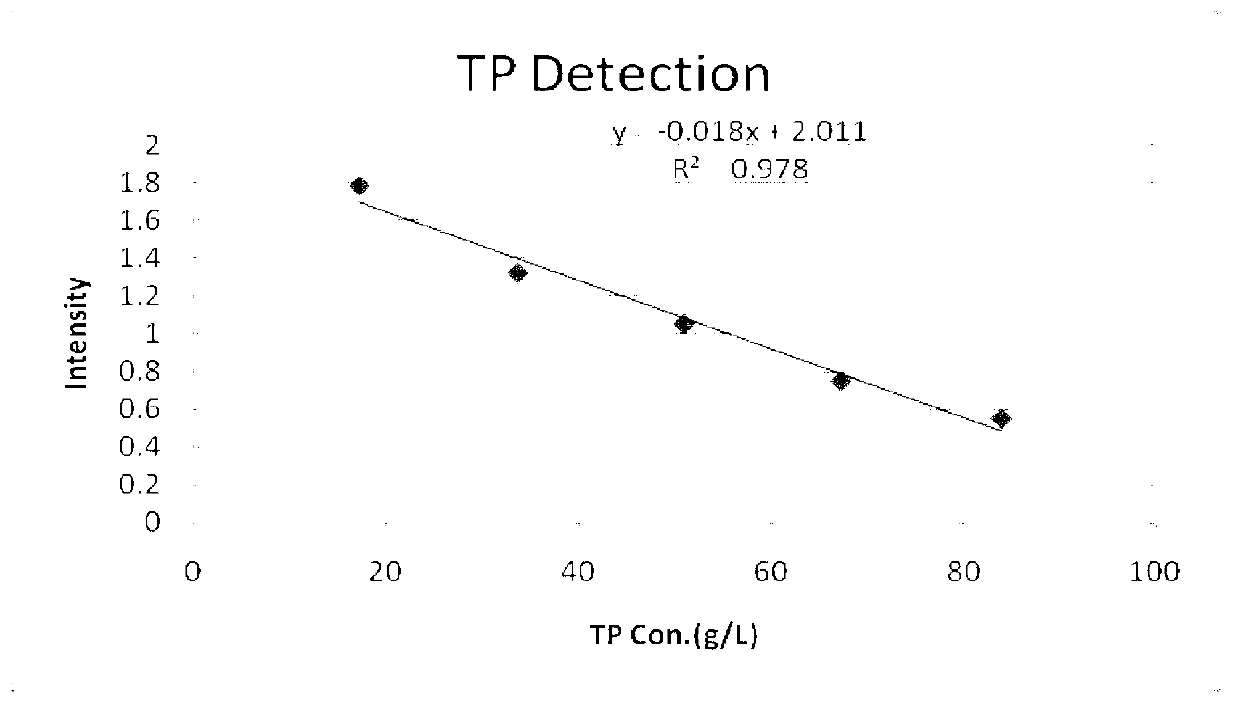
Total protein detection reagent
ActiveCN103278469AHigh sensitivityImprove accuracyColor/spectral properties measurementsVitamin CFreeze-drying
The invention discloses a total protein detection reagent which comprises a diluent and a reaction reagent, wherein the diluent is composed of trihydroxymethyl aminomethane, surfactant, preservative, anti-bilirubin interference agent and vitamin C oxidase; and the reaction reagent is composed of sodium hydroxide, copper sulfate, potassium sodium tartrate, potassium iodide and freeze-drying protective agent. The detection reagent disclosed by the invention has favorable sensitivity, accuracy, precision and linearity, and can completely satisfy the clinical examination requirements.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
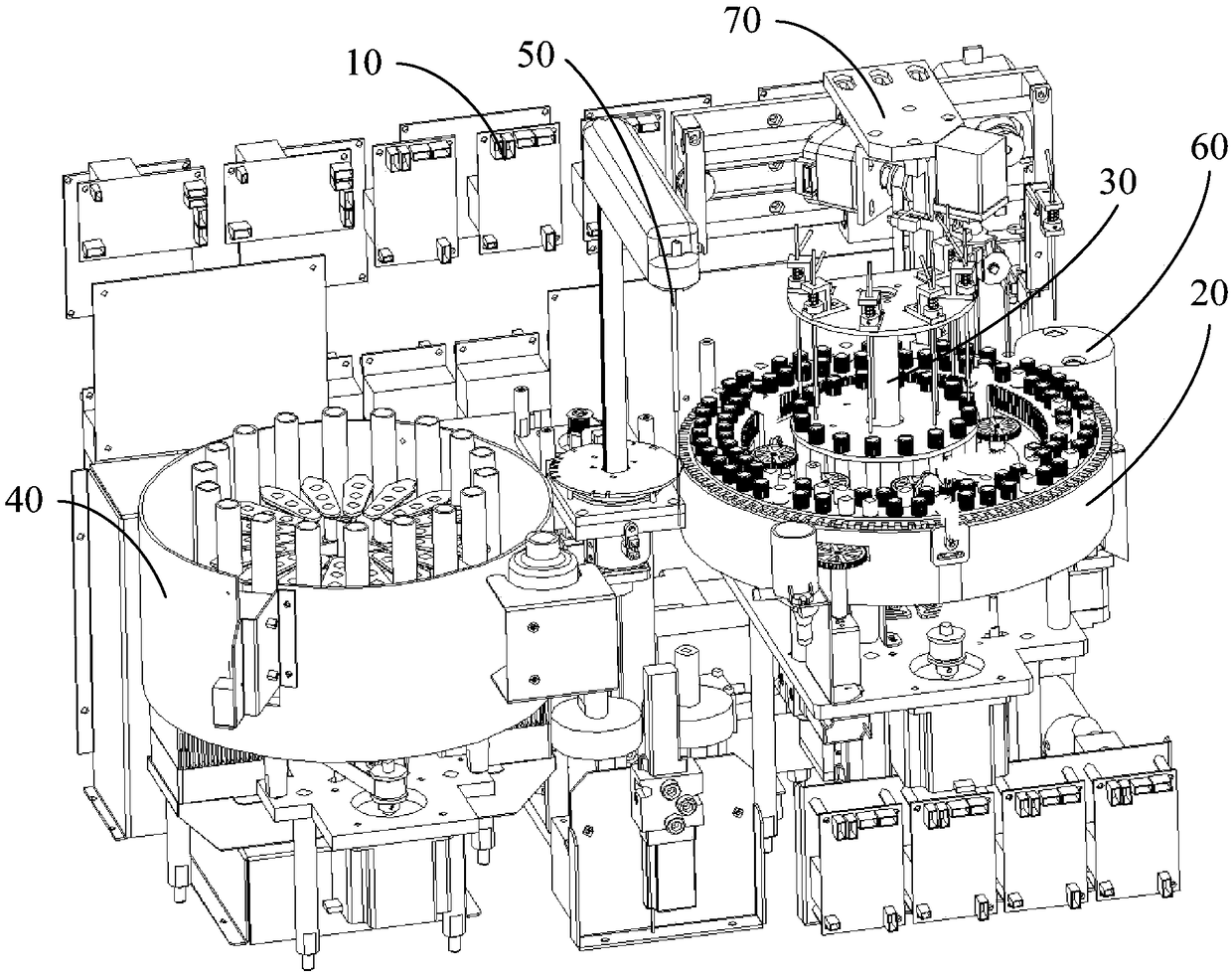
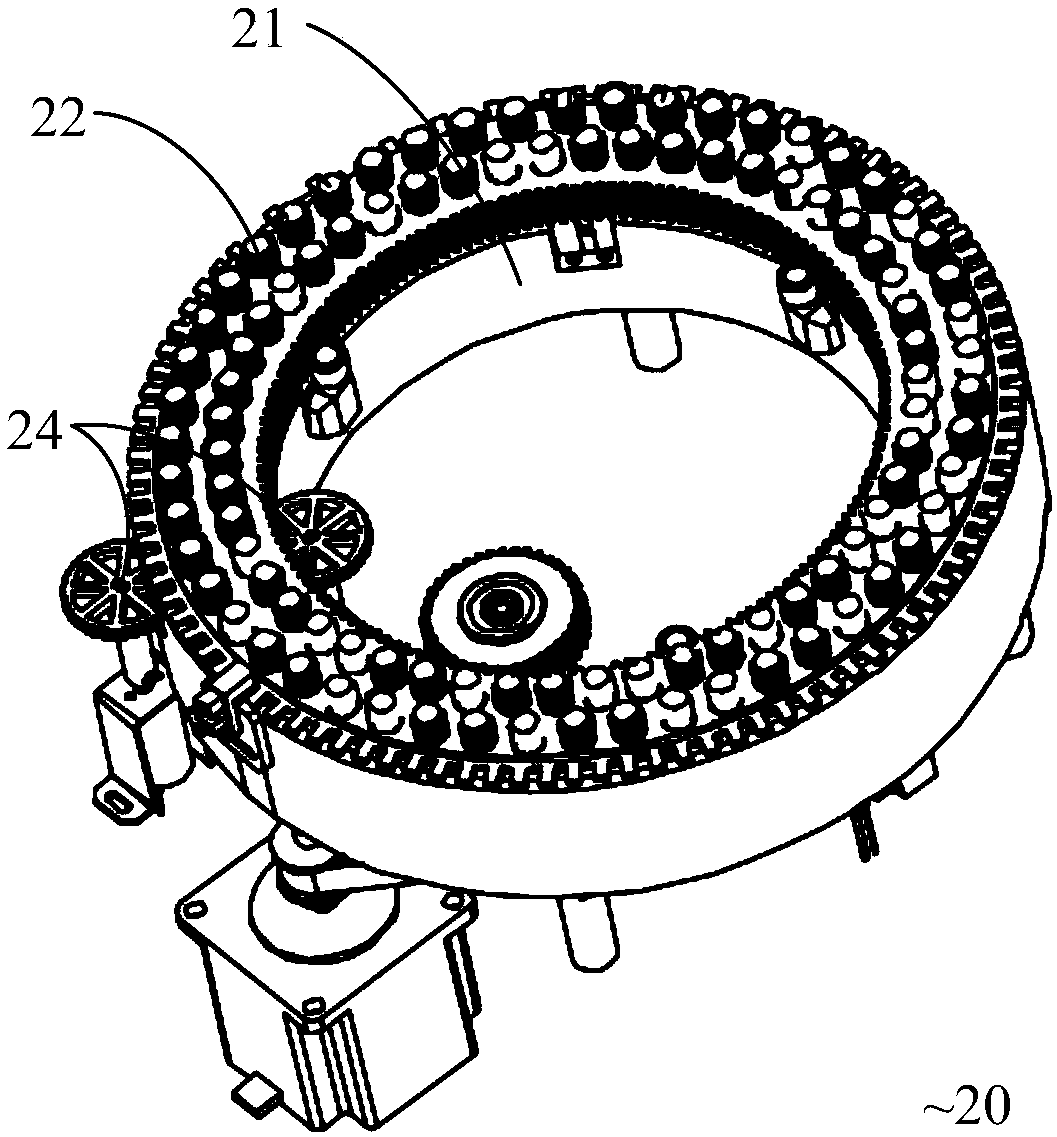
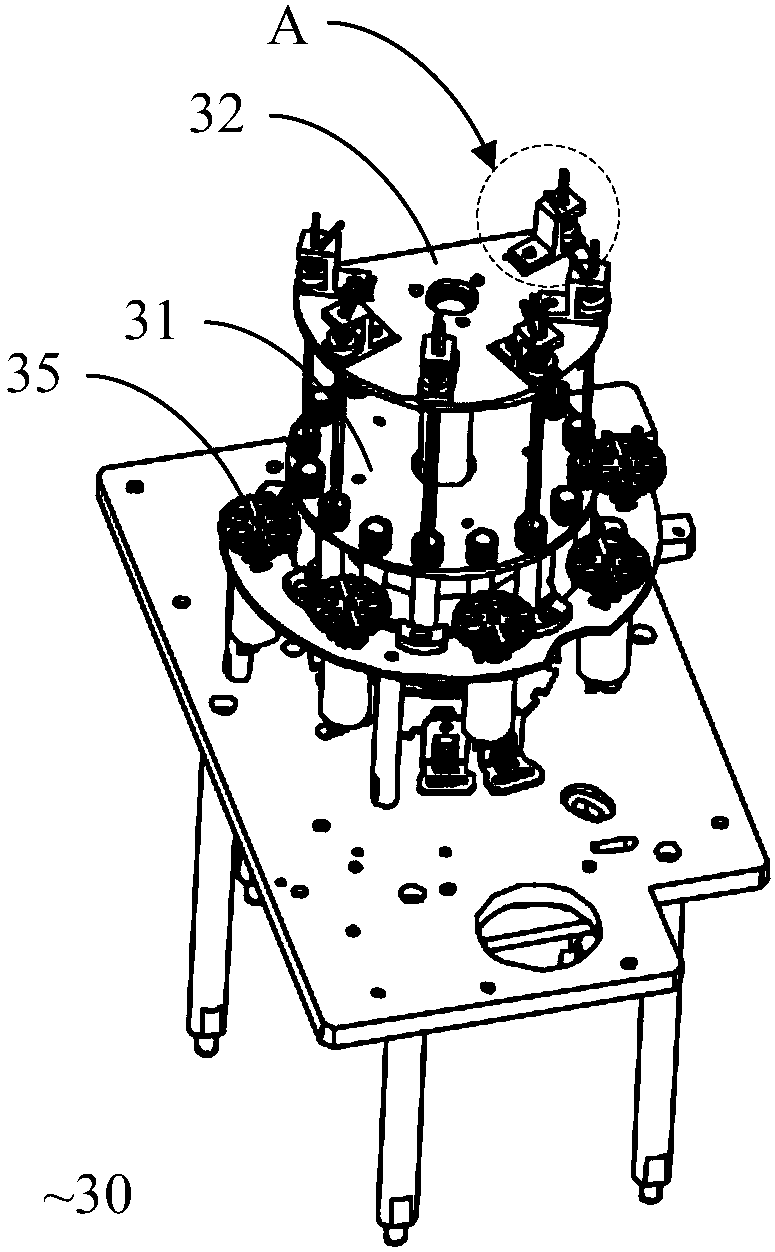
POCT full-automatic chemically luminescent immunoassay instrument
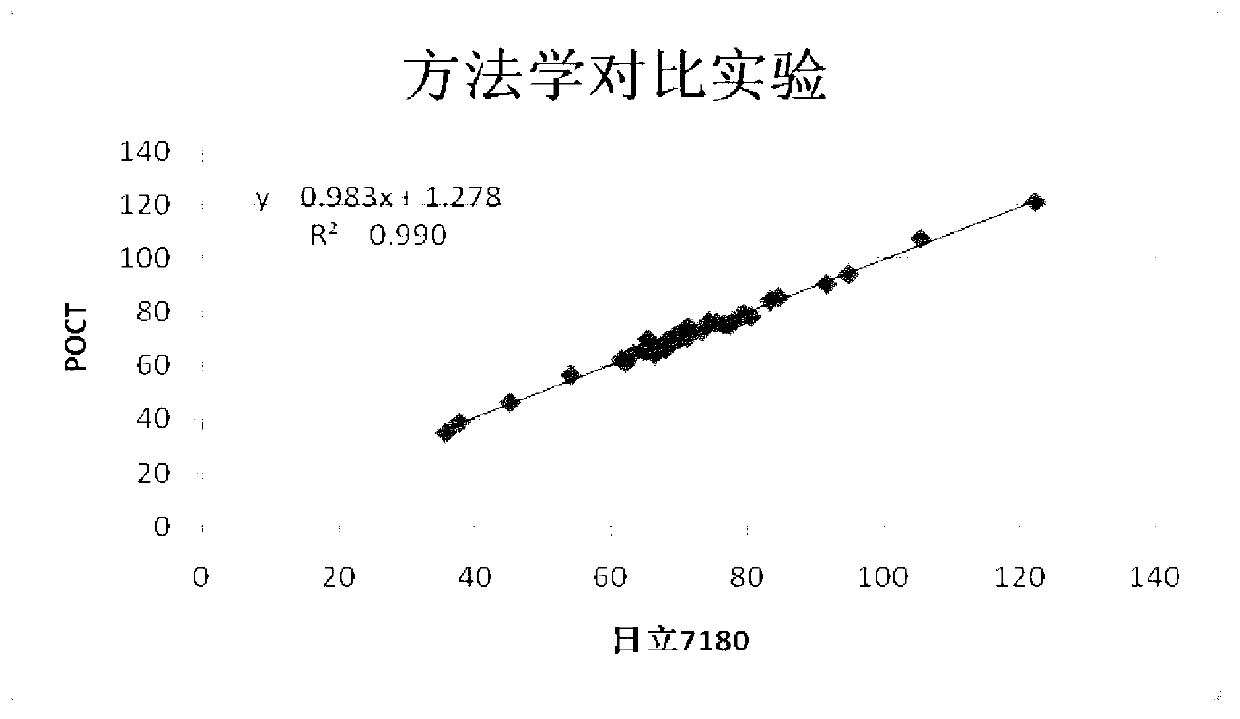
PendingCN108469529AReduce volumeMeet clinical testing requirementsMaterial analysisClinical testsAnalytical chemistry
The invention discloses a POCT full-automatic chemically luminescent immunoassay instrument. The instrument comprises a rack, a reaction mechanism, a cleaning mechanism and a reagent sample mechanismarranged corresponding to the reaction mechanism, wherein the reaction mechanism and the reagent sample mechanism are both arranged on the rack, the reaction mechanism is a hollow structure, and the cleaning mechanism is arranged in the hollow structure of the reaction mechanism and used for cleaning a reaction cup in the reaction mechanism. According to the technical scheme, a reaction disc is arranged to be a hollow structure, the cleaning mechanism is arranged in the reaction disc, so that it can be achieved that the cleaning operation can be completed in the reaction mechanism, thereby achieving the purpose of reducing the equipment size, current clinical test requirements are in accord, and the reaction cup is transmitted to an optical detection mechanism for detection after being cleaned.
Owner:深圳天辰医疗科技有限公司
Stable reagent kit of ethanol determination liquid
ActiveCN102495220AProtection stabilityMeet clinical testing requirementsColor/spectral properties measurementsBiological testingEthanol dehydrogenaseAlcohol ethyl
The invention discloses a stable reagent kit of ethanol determination liquid, which is a liquid double reagent formed by reagent I and reagent II, wherein the reagent I includes buffer solution (pH8.5-9.5)0.05-0.5M, reaction promoter 1-200g / L, stabilizer 0.1-10g / L, and preservative 0.1-10g / L; and reagent II includes buffer solution (pH6.0-7.0)0.05-0.5M, beta-coenzymeI 0.1-10g / L, alcohol dehydrogenase 0.2-200KU / L, compound stabilizer 0.1-10g / L, activator 0.1g-100g / L, and preservative 0.1-10g / L. The invention has the advantages that the reagent kit is highly accurate, precise and stable, can be stably stored at least for 12 months away from the light, and can fully satisfy the requirements of clinic detection.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Direct bilirubin detection reagent
ActiveCN103333945AHigh sensitivityImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsVitamin CFreeze-drying
The invention discloses a direct bilirubin detection reagent. The direct bilirubin detection reagent comprises diluents and reaction reagents. The diluents comprise a buffer 1, a surfactant, an antiseptic and ascorbate oxidase. The reaction reagents comprise a buffer 2, common salt, disodium ethylene diamine tetraacetate, hydroxylamine sulphate, etidronic acid, sodium persulfate, sulfuric acid, an antiseptic and a freeze-drying protective agent. The direct bilirubin detection reagent has good sensitivity, accuracy, precision and linearity and can satisfy clinical examination requirements.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Detection method and kit for detecting 25-hydroxyvitamin D (25OHD) in human serum
InactiveCN109781909AStrong interference abilityHigh precisionComponent separationSerum samplesUltrafiltration
The invention provides a detection method and a kit for detecting 25-hydroxyvitamin D (25OHD) in human serum. The method comprises the following steps: mixing a serum sample with a sodium hydroxide aqueous solution uniformly; performing ultrafiltration centrifugation; adding an isotope internal standard solution of 25-hydroxyvitamin D in the ultrafiltrate; after full and uniform mixing, adding a methyl tertiary butyl ether solvent and carrying out vortex shock extraction; after centrifugation, taking a supernatant and carrying out nitrogen drying, and carrying out redissolving to obtain a to-be-measured sample; and then carrying out detection. The detection method has advantages of simple pre treatment, high specificity and resistance to matrix interference, high detection precision and accuracy, and high detection sensitivity.
Owner:上海墨煦科技发展合伙企业(有限合伙)
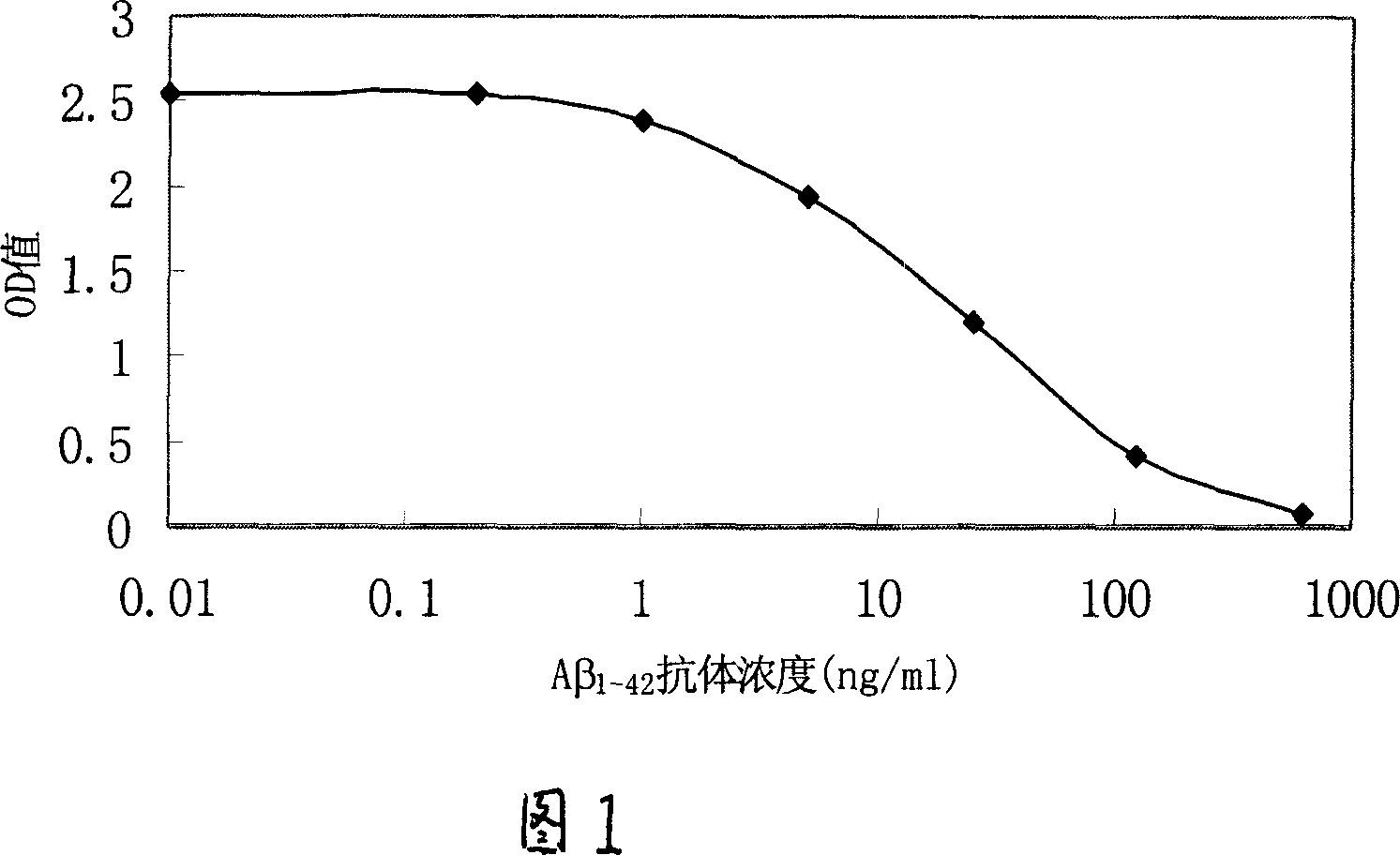
Method for determination of A-beta antibody and reagent kit therefor
InactiveCN1928564AImprove accuracyThe method is simpleMaterial analysis by observing effect on chemical indicatorAntigenBiology
The disclosed detection method for Abeta1-42 antibody comprises: covering solid holder by the Abeta1-42 protein fragment; adding the target sample into the holder to cultivate and clean the non-combined impurity; adding Abeta1-42 mono-clone antibody solution to cultivate and clean any the non-combined; detecting the content of combined component in the mixture. This invention has well accuracy and fit to quantitative detection in wide clinic application.
Owner:刘俊华
CRISPR detection primer group for bordetella pertussis and application of CRISPR detection primer group
ActiveCN110791578AShorten detection timeIncreased sensitivityMicrobiological testing/measurementMicroorganism based processesPertusariaSequenceome
The invention relates to a CRISPR detection primer group for bordetella pertussis and an application of the CRISPR detection primer group, which belong to the technical field of gene detection of CRISPR technologies. The primer group comprises an amplification primer pair and crRNA, wherein the amplification primer pair is used for amplifying a sequence of bordetella pertussis as shown in SEQ ID NO.1; wherein the crRNA comprises an anchoring sequence and a guide sequence, the anchoring sequence and the Cas protein are specifically recognized, and the guide sequence is matched with the targeting sequence fragment in the SEQ ID NO.1 sequence. By adopting the primer group, the bordetella pertussis is detected by a CRISPR technology, so that the detection time of the bordetella pertussis is shortened, and the detection can be completed within 60 minutes. According to the invention, a specific sequence combination obtained by screening is used as the primer group for detection, so that theprimer group has the advantages of high sensitivity and strong specificity, and the detection limit can reach 3 copies. The primer group is used for CRISPR detection of bordetella pertussis, dependence on qPCR instruments and other complex variable-temperature amplification instruments is avoided, and the primer group has wide application prospects.
Owner:广州微远医疗器械有限公司 +3
Primer for detecting alcohol metabolizing genes by aid of pyrosequencing joint sequencing methods and application of primer
ActiveCN106987623AGood specificityGood accuracyMicrobiological testing/measurementDNA/RNA fragmentationAlcohol dehydrogenasePyrosequencing
The invention discloses a primer for detecting alcohol metabolizing genes by the aid of pyrosequencing joint sequencing methods, and belongs to the technical field of biological detection. The primer comprises amplification primers shown as SEQ ID NO.1-4 and sequencing primers shown as SEQ ID NO.5-6. Biotin labeling is carried out by 5' ends of the primers shown as SEQ ID NO.1 and SEQ ID NO.3. The invention further discloses application of the primer to simultaneously detecting the polymorphism of SNP (single nucleotide polymorphism) loci rs671 of ALDH2 (acetaldehyde dehydrogenase 2) genes and SNP loci rs1229984 of ADH1B (alcohol dehydrogenase 1B) genes. The primer is high in detection throughput as compared with the traditional ordinary pyrosequencing with only single-SNP polymorphism detection capacity during sequencing reaction in each procedure, the detection time can be effectively shortened, and labor and the cost can be effectively reduced. Besides, the primer and the application have the advantages of accurate detection results, good specificity, high sensitivity, short detection cycle, simplicity in operation and capability of meeting clinical examination requirements.
Owner:石家庄迪安医学检验实验室有限公司
Disposable hard connecting band collection container and anti-freezing vacuum blood taking needle of bleeding opening
The invention belongs to the technical field of medical instruments, and particularly relates to a disposable hard connecting band collection container and an anti-freezing vacuum blood taking needle of a bleeding opening. The disposable hard connecting band collection container is formed in the following mode that: an anticoagulant coating layer is arranged on an inner cavity wall of a circular connector; one end of the connector is provided with a venous blood taking needle; the rear part of the connector is provided with a vacuum valve; the other end of the connector is provided with the blood collection sample bottle needle; the blood collection sample bottle needle is connected with a blood collection sample bottle cap; the blood collection sample bottle cap is connected with a rubber plug; the rubber plug is connected with a blood collection sample bottle; and one side on the blood collection sample bottle close to the blood collection sample bottle cap is provided with one secondary bleeding opening. The disposable hard connecting band collection container and the anti-freezing vacuum blood taking needle of the bleeding opening have the advantages that: the anticoagulant coating layer is added on the basis of the conventional hard connection type blood taking needle so that blood flowing out of a body can contact an anticoagulant; a blood sample is ensured to generate no cruor and hemolysis so as to ensure the accuracy of the test result; and the secondary bleeding opening is added so that a clinical inspector can flexibly control the blood dosage during the test to ensure repeated tests at any time or add new testing items.
Owner:天津百新生物技术研发有限公司
Leucine aminopeptidase detection reagent
ActiveCN103266164AGood sensitivityEasy to operateMicrobiological testing/measurementChemistryFreeze dry
The invention discloses a leucine aminopeptidase detection reagent which is characterized by comprising a diluent and a reaction reagent, wherein the diluent is composed of a buffer solution, a surfactant, a preservative, a bilirubin interference remover and a vitamin C oxidase; and the reaction reagent is composed of a buffer solution, sodium chloride, L-leucine-4-nitroaniline, 0.1-1 g / L preservative and 10-30 g / L freeze-drying protective agent. The detection reagent disclosed by the invention has favorable sensitivity, accuracy, precision and linearity, and can completely satisfy the clinical examination requirements.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Ventricular fibrillation detecting genetic differential diagnosis kit
InactiveCN108300778ALow costImprove capture efficiencyMicrobiological testing/measurementExonVentricular fibrillation
The invention discloses a ventricular fibrillation detecting genetic differential diagnosis kit. The ventricular fibrillation detecting genetic differential diagnosis kit is characterized by being a genetic differential diagnosis kit for detecting ventricular fibrillation by a massively parallel sequencing platform NGS technology. The ventricular fibrillation detecting genetic differential diagnosis kit has the main characteristics as follows: A, capture probes are uniquely designed and prepared for all exon fragments of 91 genes; B, unique connectors with label sequences are designed; C, universal primers perform PCR amplification on probe sequences; D, unique operation of capturing mixed target segments is designed.
Owner:FUWAI HOSPITAL CHINESE ACAD OF MEDICAL SCI & PEKING UNION MEDICAL COLLEGE
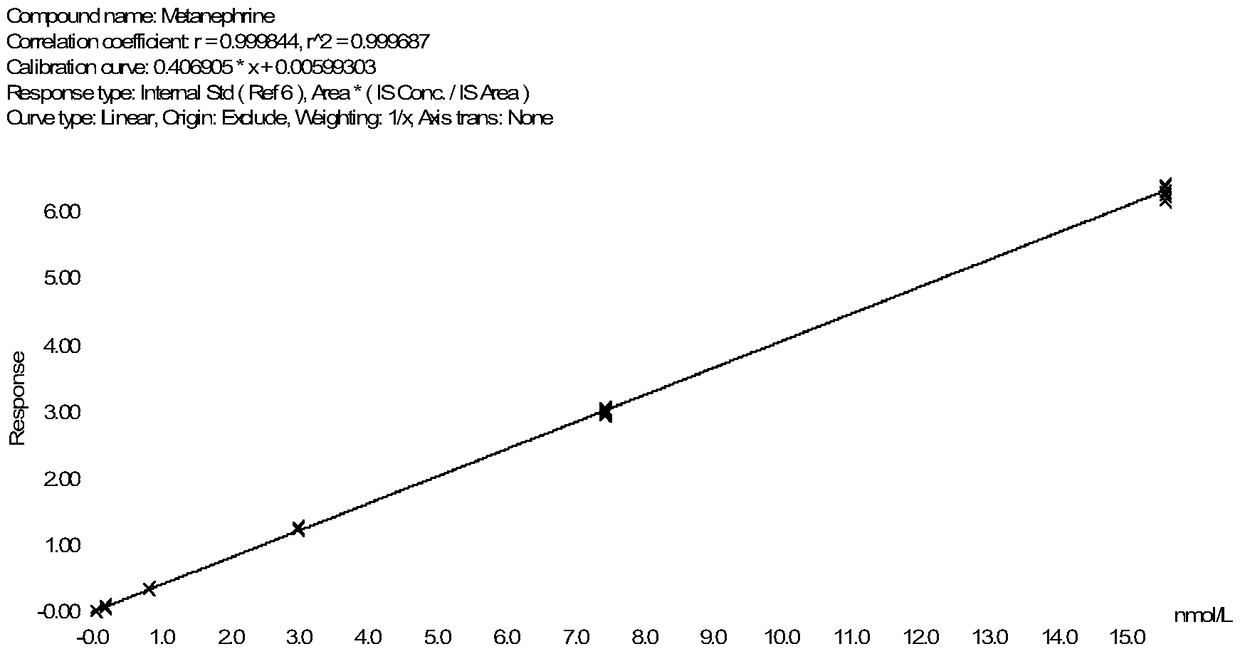
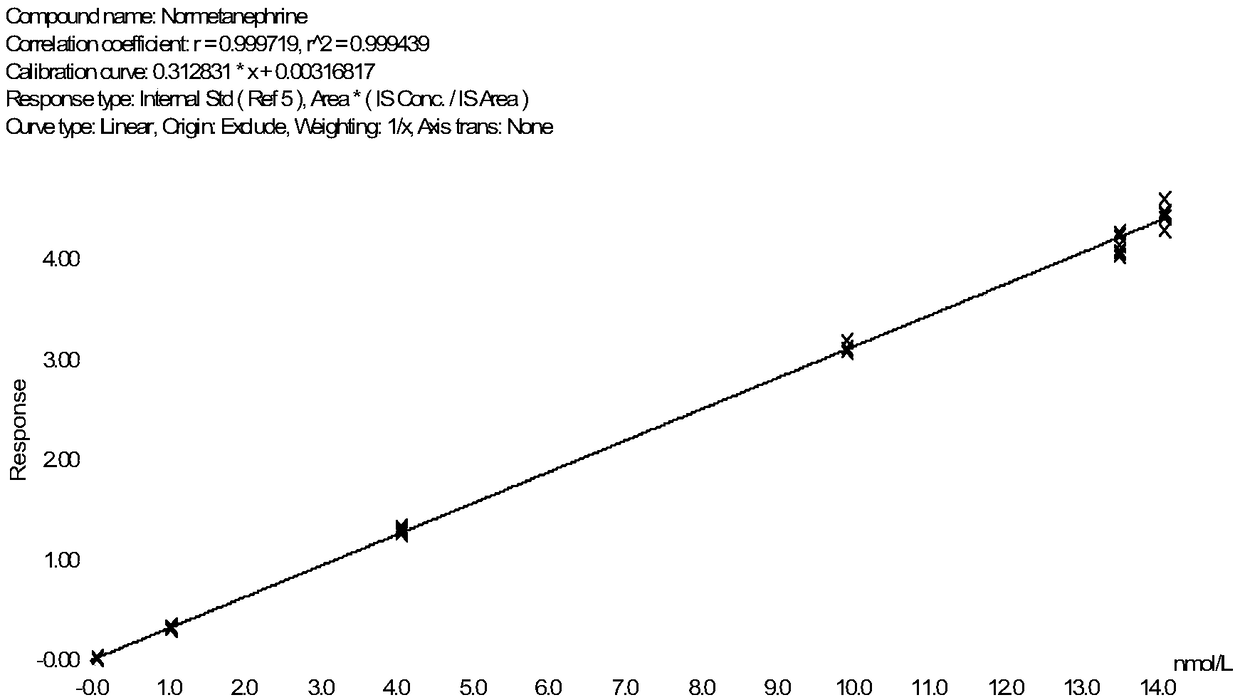
Method for detecting metanephrines through liquid chromatography-tandem mass spectrometry
The invention discloses a method for detecting metanephrines through liquid chromatography-tandem mass spectrometry. The method comprises the following steps that 1 sampling preprocessing is conducted, wherein a mixed internal standard solution is added into a to-be-detected sample for protein precipitation, then centrifugation is conducted to acquire a liquid supernatant, and the liquid supernatant is blow-dried with nitrogen gas and then subjected to double solving by using a double solvent to obtain a double solution; 2, the double solution obtained in the step 1 is purified; and 3, the metanephrines in a purified product obtained in the step 2 are determined by means of liquid chromatography-tandem mass spectrometry. The method is simple in pre-processing, high in sensitivity and specificity and good in repeatability, part of artificial operations are replaced with on-line automatic pre-processing, the detection efficiency is improved, the error rate is decreased, the requirement on detection personnel is lowered, the cost is reduced, and the method is suitable for being applied and popularized in a clinical chromatography-mass spectrometry laboratory with the low automation degree.
Owner:GUANGZHOU KINGMED DIAGNOSTICS CENT
Reagent kit using flow sealing technique for immuno-gold label detection
InactiveCN1338632ASimple manufacturing processReduce sensitivityBiological testingAntigenEngineering
A reagent kit using flow closing technique for immuno-gold label detection features that after the specimen to be tested is added to the specimen hole on detection board or the test strip is insertedin the specimen bath, the chromatographic moving of specimen drives the closing agent of specimen pad or colloidal gold pad to nitrocellulose film, so performing seal in flow procedure. Its advantages include simplified process, low cost, no damage to nitrocellulose film and high sensitivity.
Owner:GENETECH BIOTECH SHANGHAI
Familial deteriorating atrioventricular conduction block gene diagnosis kit
InactiveCN107937510AImprove capture efficiencyLow costMicrobiological testing/measurementAtrioventricular Conduction BlockExon
The invention discloses a familial deteriorating atrioventricular conduction block gene diagnosis kit, and is characterized in that the invention relates to the diagnosis kit for detecting familial deteriorating atrioventricular conduction block genes by a large-scale parallel sequencing platform NGS technique. The kit is mainly characterized in that: A, a capture probe is uniquely designed and prepared and aims at all exon fragments of genes SCN5A and TRPM4; B, a connector with a label sequence is uniquely designed; C, a probe sequence is subjected to PCR amplification by universal primers; and D, a uniquely designed target fragment after mixing is subjected to capture operation.
Owner:FUWAI HOSPITAL CHINESE ACAD OF MEDICAL SCI & PEKING UNION MEDICAL COLLEGE
Primer and method for detecting polymorphism of VKORC1 and CYP2C9 genes by adopting pyrosequencing method
PendingCN107201407AStrong specificityAccurate distinctionMicrobiological testing/measurementDNA/RNA fragmentationA-DNAGel electrophoresis
The invention discloses a primer and a method for detecting polymorphism of VKORC1 and CYP2C9 genes by adopting a pyrosequencing method, and belongs to the field of molecular biology examination. The primer comprises amplification upstream and downstream primers and sequencing primers of genes including VKORC1-1639G being more than A, CYP2C9430C being more than T, and CYP2C91075A being more than C. The method comprises specimen collection and DNA extraction, PCR (polymerase chain reaction) amplification reaction, gel electrophoresis on a PCR product and pyrosequencing, wherein a DNA extraction kit used in the specimen collection and DNA extraction step is prepared from lysis buffer liquid, adsorbent, rinsing buffer liquid and elution buffer liquid; the lysis buffer liquid is prepared from sodium chloride, sodium citrate, a denaturing agent, a reducing agent, ethylenediamine tetraacetic acid, ionic surfactant, absolute ethyl alcohol and ultra-pure water. In summary, the detection method provided by the invention has the advantages of an accurate detection result, high specificity, a short detection period, a simple operation, low cost and the like.
Owner:GUIZHOU PROVINCIAL PEOPLES HOSPITAL
Sickbed control device and automatic control method for sickbed
InactiveCN106901928AReliable completionReduce or even eliminate uncertaintiesNursing bedsAutomatic controlPassive leg raising test
The invention discloses a sickbed control device. The sickbed control device is characterized by comprising a bedstead body, a first movable bed plate, a middle plate, a second movable bed plate, a first electric push rod, a second electric push rod, a first position sensor, a second position sensor and a controller, wherein the first position sensor and the second position sensor are used for detecting movement positions of the first movable bed plate and the second movable bed plate separately; when the first movable bed plate or the second movable bed plate moves to the preset position, transmission information is fed back to the controller. The invention further provides an automatic control method for a sickbed. According to the sickbed control device and the automatic control method for the sickbed, the state of the sickbed is quantified, uncertain factors in a test are reduced or even removed, and a passive leg raising test is automatically completed accurately step by step according to preset parameters of a user. According to the automatic control method for the sickbed, medical workers can be better assisted in safely and efficiently completing the passive leg raising test at high quality.
Owner:GUANGZHOU MEDSOFT SYST LTD
Kit for examining Liddle's related gene mutation
InactiveCN104087657ALow costImprove capture efficiencyMicrobiological testing/measurementLiddle's syndromeGenes mutation
The invention relates to a kit made for detecting Liddle's syndrome related gene mutation samples, and concretely relates to a product for detecting Liddle's syndrome related gene by adopting a large scale parallel sequencing platform technology. A detection method comprises the following steps: 1, uniquely designing and making a capture probe against all exon fragments of gene SCNN1B and SCNN1G; 2, designing a unique joint with a labeling sequence; 3, carrying out PCR amplification on the sequence of the probe by using a general primer; and 4, designing a unique capture operation of mixed target fragments. The method and the kit have the advantages of large flux of prepared large scale parallel sequencing platform samples, high efficiency, simple operation, and great reduction of the sequencing examination cost.
Owner:百世诺(北京)医疗科技有限公司
Differential diagnostic kit for myocardial hypertrophy genes
InactiveCN108034717ALow costImprove capture efficiencyMicrobiological testing/measurementDNA/RNA fragmentationCardiac muscleTNNT2
The invention discloses a differential diagnostic kit for myocardial hypertrophy genes. The differential diagnostic kit is characterized that the technology relates to the differential diagnostic kitfor detecting the myocardial hypertrophy genes by applying a large-scale parallel sequencing platform NGS (Next-Generation Sequencing) technique. The differential diagnostic kit is mainly characterized in that A, unique design and preparation of capture probes aiming at all exon fragments of genes such as ACTC1, ACTN2, ANKRD1, CALR3, CASQ2, CAV3, CSRP3, FHL1, JPH2, LDB3, MYBPC3, MYH6, MYH7, MYL2,MYL3, MYLK2, MYOZ2, MYPN, NEXN, PLN, PRKAG2, SLC25A4, TCAP, TNNC1, TNNI3, TNNT2, TPM1, TRIM63, TTN, VCL, GLA, TTR, LAMP2 and FLNC; B, unique design of a connector with a tag sequence; C, PCR (Polymerase Chain Reaction) amplification of the probe sequence by using a universal primer pair; D, capture operation of mixed target fragments with unique design.
Owner:FUWAI HOSPITAL CHINESE ACAD OF MEDICAL SCI & PEKING UNION MEDICAL COLLEGE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com