MULTI-BRANCHED CATIONIC PHOSPHONIUM SALT and FORWARD OSMOSIS EXTRACT CONTAINING THE SAME
A forward osmosis and extraction solution technology is applied in the field of multi-branched cationic salts and forward osmosis extraction solutions containing the same, and can solve the problems of difficulty in removing magnetic nanoparticles, high energy consumption, and difficulty in redispersion of magnetic particles.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
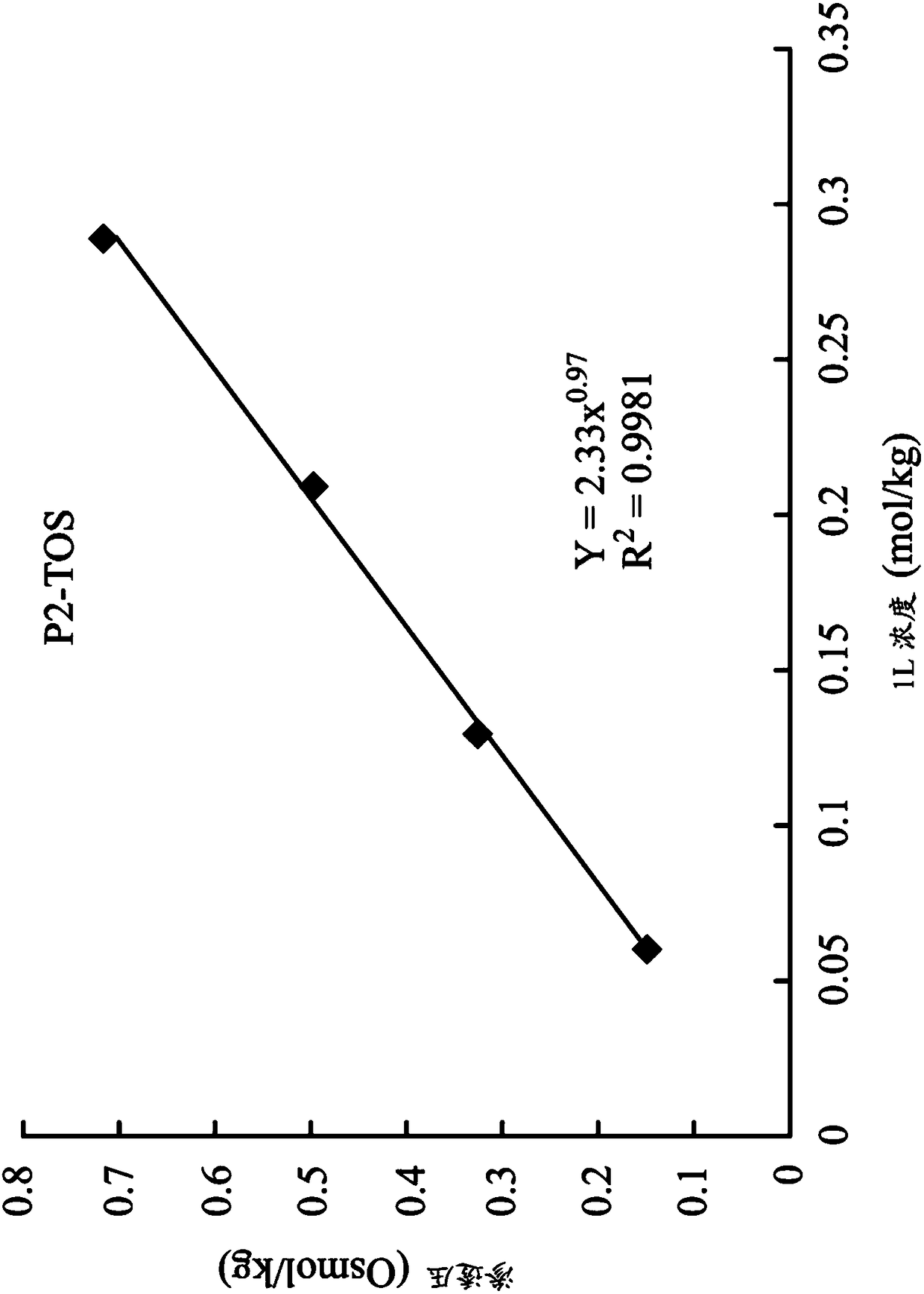
[0060] 1,8-octanediyl-bis(tri-n-butyl ) bis(p-toluenesulfonate) (P2-TOS)
[0061] First, synthesize 1,8-octanediyl-bis(tri-n-butyl ) dibromide (1,8-octanediyl-bis (tri-n-butylphosphonium) dibromide) (hereinafter referred to as P2-Br):
[0062] Take a 500mL round bottom bottle, put 80g (0.4mol) of tributylphosphine (tributylphosphine) and 48.9g (0.18mol) of 1,8-dibromooctane (1,8-dibromooctane), and then add 150mL of anhydrous acetone, Stir at 40°C for 48 hours.
[0063] After the reaction was over, the above solution was slowly dropped into 1.5L of ether. The resulting white powder solid was filtered and washed several times with ether.
[0064] The washed white solid was dried to obtain 117 g of the product P2-Br.
[0065] Next, synthesize 1,8-octanediyl-bis(tri-n-butyl ) two (p-toluenesulfonate) (1,8-octanediyl-bis (tri-n-butylphosphonium) di (p-toluenesulfonate)) (hereinafter referred to as P2-TOS):
[0066] 2.67 g (3.7 mmol) of P2-Br and 1.57 g (8.1 mmol) of sodi...
preparation example 2
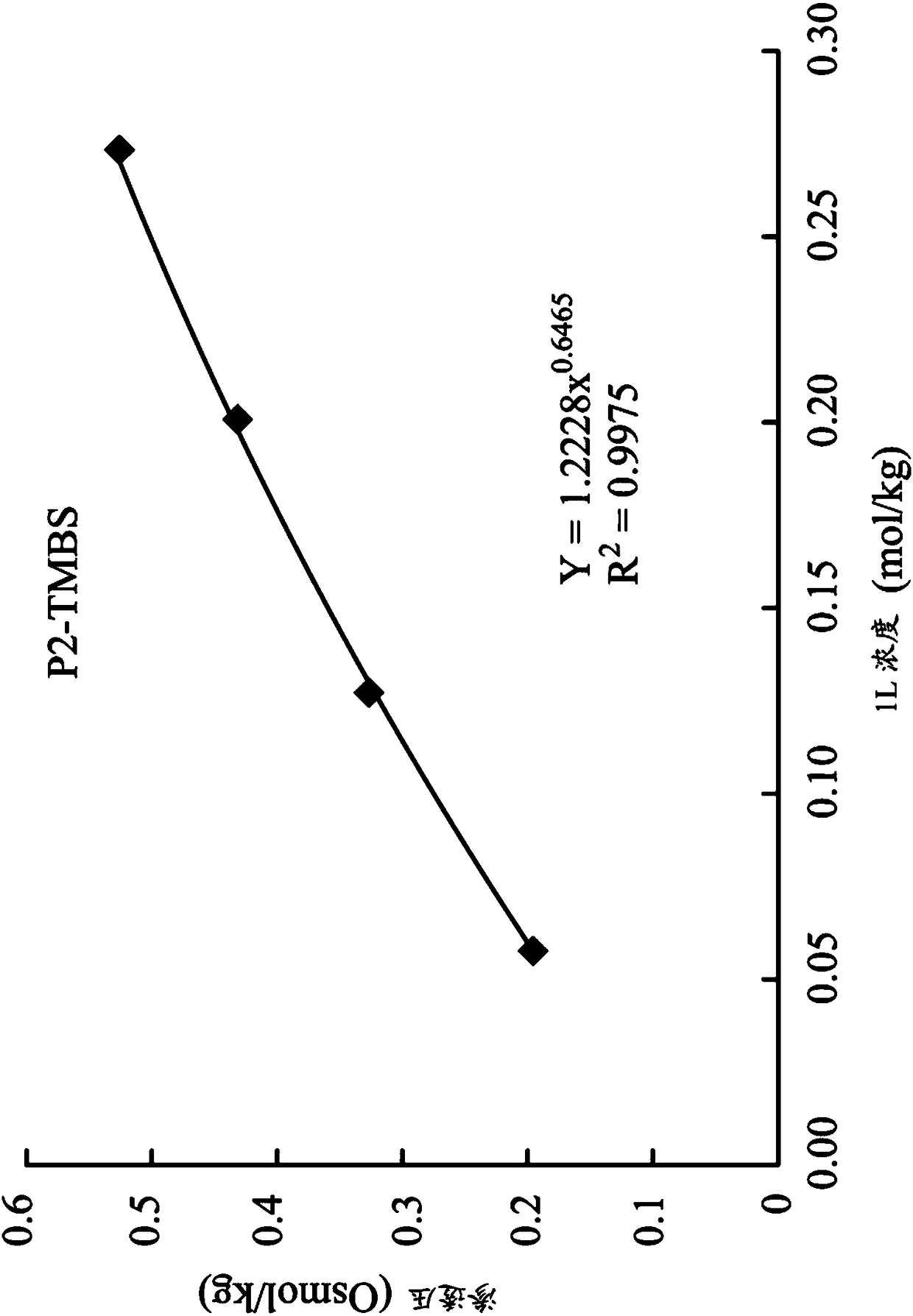
[0072]1,8-octanediyl-bis(tri-n-butyl ) Bis(2,4,6-trimethyl-benzenesulfonate) (P2-TMBS)
[0073] Synthesis of 1,8-octanediyl-bis(tri-n-butyl ) Di(2,4,6-trimethyl-benzenesulfonate) (1,8-octanediyl-bis(tri-n-butylphosphonium)di(2,4,6-trimethyl-benzenesulfonate)) (hereinafter referred to as P2 -TMBS):
[0074] Take P2-Br 10g (14.7mmol) and 2,4,6-trimethylbenzenesulfonate (sodium2,4,6-trimethyl-benzenesulfonate) (TMBS-Na) 6.8g (29.6mmol) dissolved in 40g of deionized water, stirred at room temperature for 24 hours.
[0075] After the reaction was completed, 20 mL of ethyl acetate was added for extraction.
[0076] The organic layer was concentrated to obtain about 12.4 g of the product P2-TMBS.
[0077] The product P2-TMBS was determined via NMR ( 1 H-NMR, 400MHz in D 2 O):0.8(t,18H,C H 3 CH 2 -), 1.09(m,4H,-CH 2 -), 1.1~1.5(m,32H,-CH 2 -), 1.9~2.0(t,16H,PCH 2 -), 2.12(s,6H,Ar-CH 3 ), 2.25(s,12H,Ar-CH 3 ), 6.88 (s, 4H, ArH). The chemical formula of the product P2-...
preparation example 3
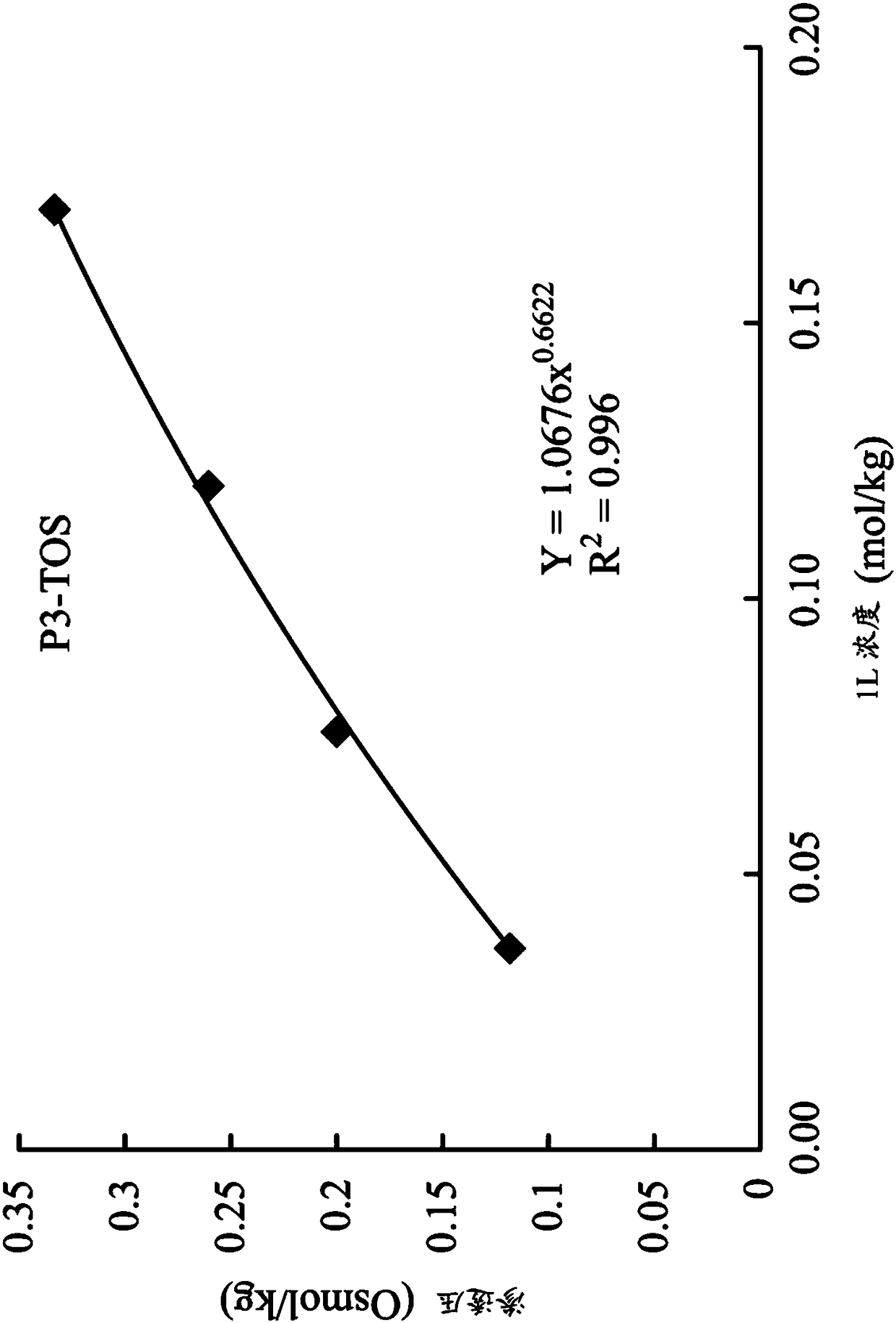
[0080] Trimethylolpropane tris[(tri-n-butyl ) butyrate] three (p-toluenesulfonate) (P3-TOS)
[0081] First, synthesize trimethylolpropane tris(4-bromobutyrate) (trimethylolpropane tris(4-bromobutyrate)):
[0082] Take a 50mL round-bottomed reaction bottle, put 1g (8.3mmol) of trimethylolpropane (trimethylolpropane), dissolve it with 20mL of anhydrous tetrahydrofuran (tetrahydrofuran; THF), then slowly add 1.1g of NaH (60%), at room temperature After stirring with a magnet for 2 hours, 5 g (27 mmol) of 4-bromobutyryl chloride (4-bromobutyryl chloride) was added dropwise and reacted overnight at room temperature.
[0083] After the reaction was completed, THF was drained, 20 mL of diethyl ether was added, the solution was filtered to remove solids, the obtained filtrate was washed three times with 50 mL of water, and then the product was drained to obtain 3.4 g of the product trimethylolpropane tri(4- bromobutyrate).
[0084] Next, synthesize trimethylolpropane tri[(tri-n-bu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com