Preparation method of pseudoephedrine hydrochloride sustained-release preparation
A technology of pseudoephedrine hydrochloride and slow-release preparations, which is applied in the direction of pharmaceutical formulas, medical preparations with no active ingredients, and medical preparations containing active ingredients, etc. It can solve problems such as unfavorable oral administration, large amount of excipients, and changes in drug release. Good slow-release function, high production efficiency and moderate dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
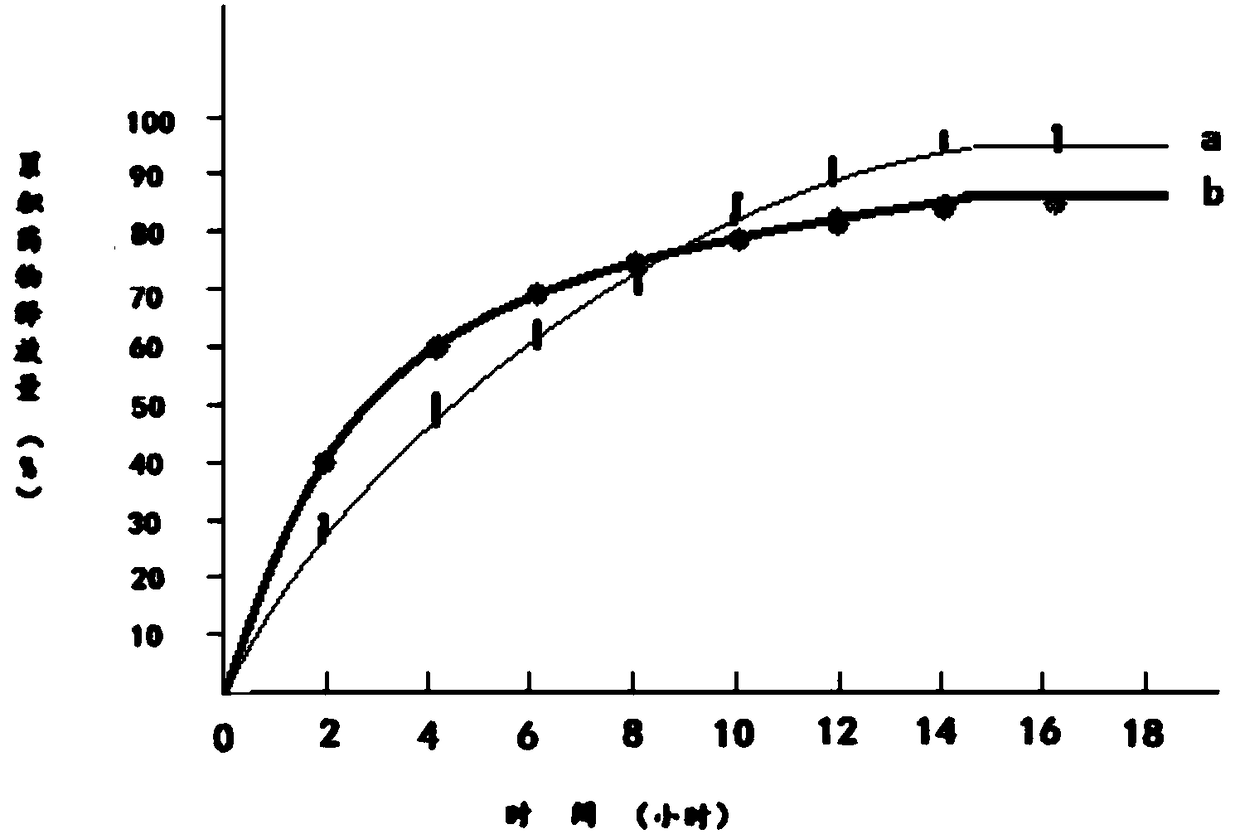
Embodiment 1
[0023] 1) Dissolve 100 g of pseudoephedrine hydrochloride in 120 g of 95% ethanol to obtain an ethanol solution of pseudoephedrine hydrochloride, which is recorded as solution A;
[0024] 2) Add 120g of hydroxypropyl β-cyclodextrin and 20g of mannitol to solution A to obtain solution B;
[0025] 3) Dissolve 120g of polylactic acid and 20g of polyethylene glycol 200 in 120g of acetone to obtain a solution of polylactic acid and polyethylene glycol 200, which is recorded as solution C;
[0026] 4) Mix solution B and solution C to obtain solution D;
[0027] 5) Transfer solution D to a magnetic stirrer, control the temperature of solution D at 15℃~18℃, continuously stir solution D for 12 hours, and then reduce the temperature of solution D to 0℃~1℃ within 2 hours and let it stand for 12 Keep the temperature of solution D at 0℃~1℃ during standing;
[0028] 6) Heat the solution D obtained in step 5, and when the temperature of the solution D rises to 15°C-18°C, continue stirring for 12 hour...
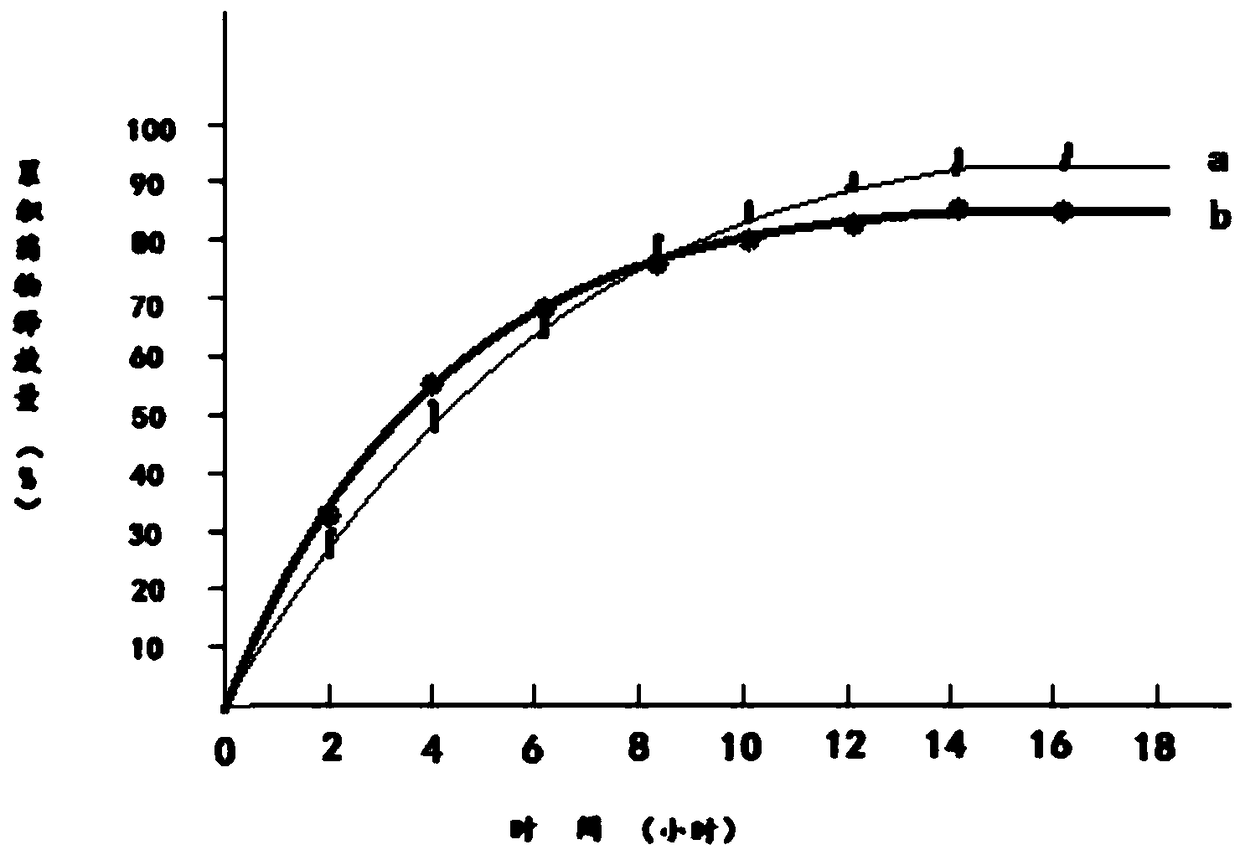
Embodiment 2
[0032] 1) Dissolve 100 g of pseudoephedrine hydrochloride in 125 g of 95% ethanol to obtain an ethanol solution of pseudoephedrine hydrochloride, which is recorded as solution A;
[0033] 2) Add 125g of hydroxypropyl β-cyclodextrin and 25g of mannitol to solution A to obtain solution B;
[0034] 3) Dissolve 135g polylactic acid and 30g polyethylene glycol 200 in 135g acetone to obtain a solution of polylactic acid and polyethylene glycol 200, which is recorded as solution C;
[0035] 4) Mix solution B and solution C to obtain solution D;
[0036] 5) Transfer solution D to a magnetic stirrer, control the temperature of solution D at 15℃~18℃, continuously stir solution D for 12 hours, and then reduce the temperature of solution D to 0℃~1℃ within 2 hours and let it stand for 12 Keep the temperature of solution D at 0℃~1℃ during standing;
[0037] 6) Heat the solution D obtained in step 5, and when the temperature of the solution D rises to 15°C-18°C, continue stirring for 12 hours, and th...
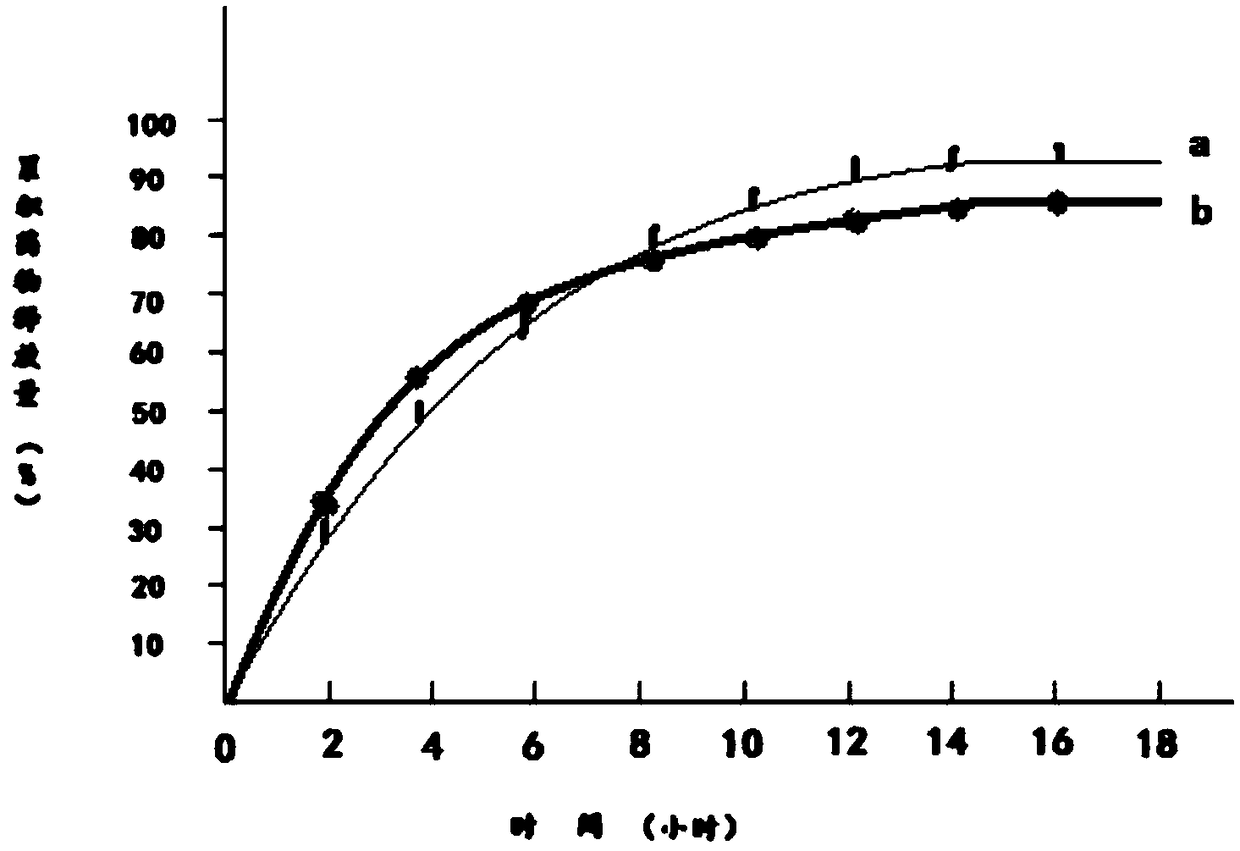
Embodiment 3
[0041] 1) Dissolve 100g of pseudoephedrine hydrochloride in 130g of 95% ethanol to obtain an ethanol solution of pseudoephedrine hydrochloride, which is recorded as solution A;
[0042] 2) Add 130g of hydroxypropyl β-cyclodextrin and 30g of mannitol to solution A to obtain solution B;
[0043] 3) Dissolve 150 g of polylactic acid and 40 g of polyethylene glycol 200 in 150 g of acetone to obtain a solution of polylactic acid and polyethylene glycol 200, which is recorded as solution C;
[0044] 4) Mix solution B and solution C to obtain solution D;
[0045] 5) Transfer solution D to a magnetic stirrer, control the temperature of solution D at 15℃~18℃, continuously stir solution D for 12 hours, and then reduce the temperature of solution D to 0℃~1℃ within 2 hours and let it stand for 12 Keep the temperature of solution D at 0℃~1℃ during standing;
[0046] 6) Heat the solution D obtained in step 5, and when the temperature of the solution D rises to 15°C-18°C, continue stirring for 12 hou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com