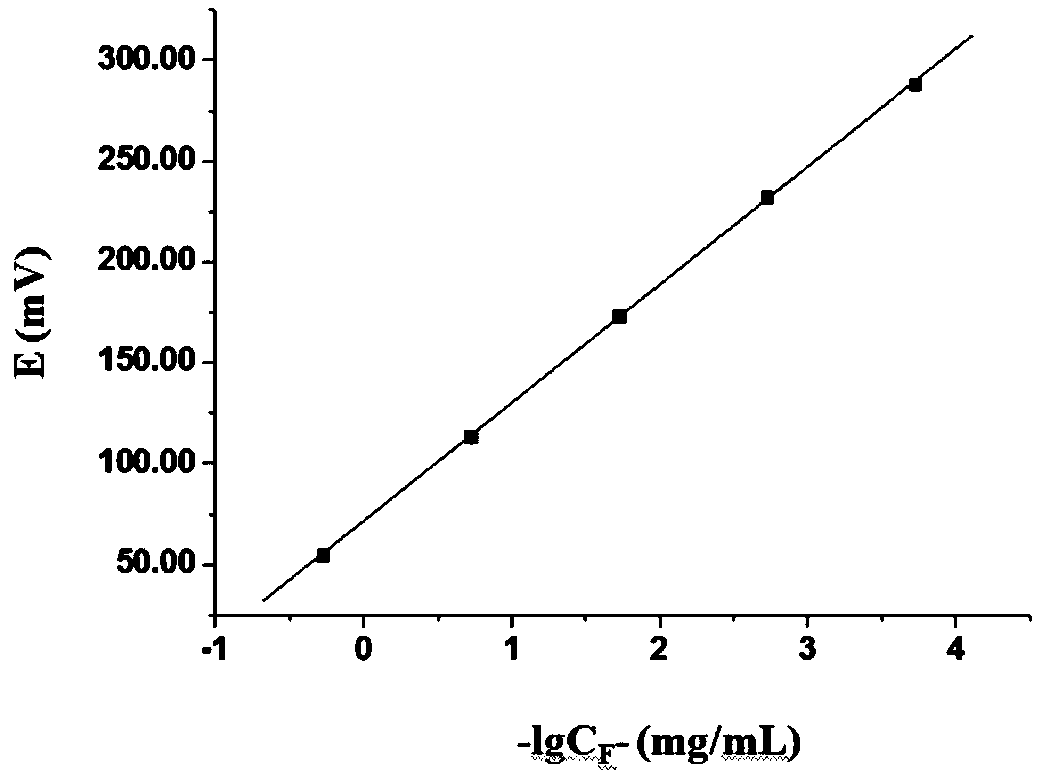Use of bifunctional metal chelate resin adsorbent in fluoride removal from wastewater
A technology of chelating resin and bifunctional groups, which is applied in the direction of adsorption of water/sewage treatment, water pollutants, water/sewage treatment, etc. It can solve the problems of inability to remove fluoride with chelating functional groups and low efficiency of fluorine removal, and achieve simple operation, Remarkable defluorination effect and short process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
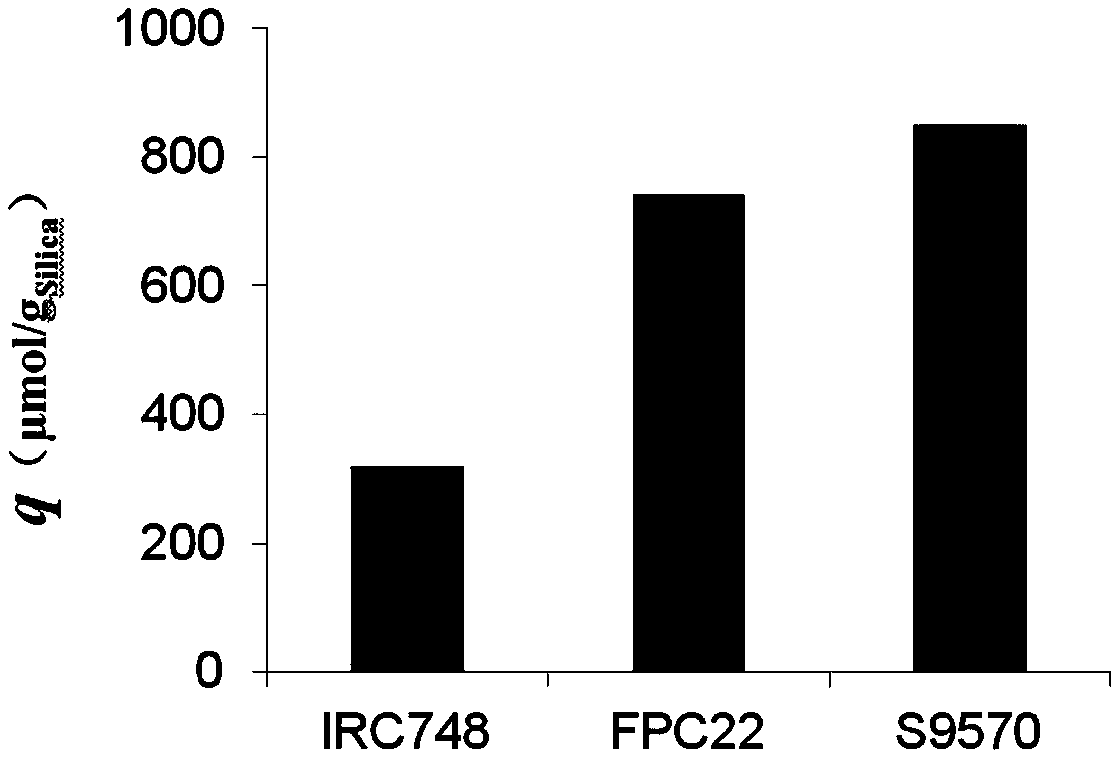
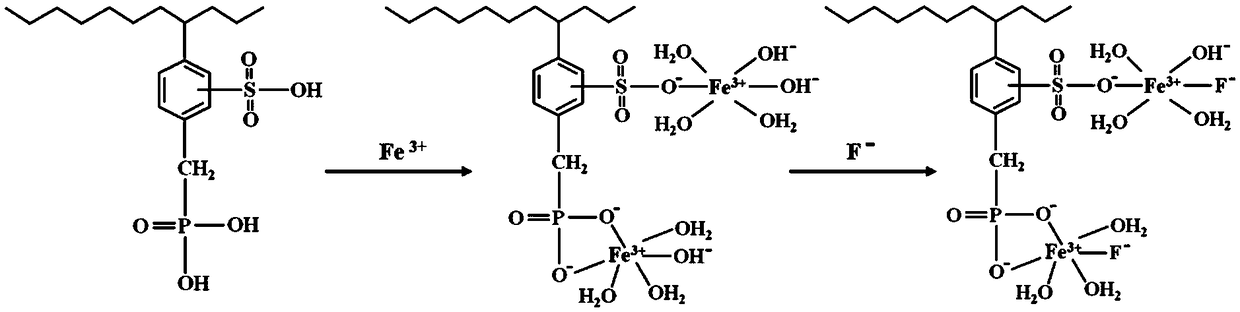
[0025] Example 1 Metal Fe 3+ Comparative Experiment of Adsorption on Chelating Resin
[0026] 1, the preparation of Fe (III) type chelating resin
[0027] The metal ions of the three chelating resins S9570, FPC22, and IRC748 were fixed according to the following steps: soak in distilled water for 12 hours to make them swell completely, and then wash repeatedly with distilled water until no floating objects can be seen and are clear and translucent; Wash with ultrapure water for 5-10 times, then put the cleaned resin into a glass column of a certain size; add 0.05mol / L FeCl at a flow rate of 2BV / h 3 The solution begins to adsorb metal ions; after the adsorption is completed, continue to add ultrapure water to wash away the residual Fe on the surface of the resin 3+ . After the resin has been cleaned after transformation, use a suction filter to remove excess water, and then dry it in an oven at 75°C for more than 36 hours.
[0028] 2. Metal Fe 3+ Determination of adsorptio...
Embodiment 2
[0040] Embodiment 2 adopts potential analysis method to draw the standard curve of fluorine content and electromotive force
[0041] 1. Preparation of standard solution
[0042] Accurately weigh 0.8400g of analytically pure NaF, dry in an oven at 150°C for 2h, dissolve in ultrapure water, dilute to 100mL, store in a polyethylene bottle, and obtain a NaF solution with a concentration of 2×10 -1 mol / L standard solution. Using this as the mother solution, serially dilute 10 times to obtain 2×10 -2 mol / L, 2×10 -3 mol / L, 2×10 -4 mol / L, 2×10 -5 mol / L series concentration of fluoride standard solution.
[0043] 2. TISAB preparation
[0044] Accurately weigh 59.0050g of analytically pure sodium chloride and 3.4830g of trisodium citrate, dissolve them in an appropriate amount of ultrapure water, add 57mL of glacial acetic acid, stir well, adjust the pH to 5.1 with 10mol / L NaOH, and dilute to 1000mL.
[0045] 3. Standard curve detection
[0046] Take 20mL standard solutions of 5...
Embodiment 3
[0047] The comparative experiment of embodiment 3 fluorides on Fe (III) type chelating resin adsorption capacity
[0048] 1. Preparation of fluorine-containing waste water simulated liquid
[0049] Accurately weigh 0.2210g of analytically pure sodium fluoride, dry in an oven at 105°C for 2h, then dissolve and dilute to 100mL, and store in a polyethylene bottle. Take 15mL fluoride standard stock solution, dilute it to 1000mL, and store it in a polyethylene bottle. This solution contains 15mg / L of fluorine, and this solution is used as the simulated feed solution of fluorine-containing wastewater.
[0050] 2. Fe(III) type chelating resin S9570-(Fe 3+ ) 2 、IRC747-Fe 3+ and FPC22-Fe 3+ Static adsorption experiment of fluoride in wastewater
[0051] Accurately take the Fe(III) type chelating resin S9570-(Fe) mentioned in embodiment 1 3+ ) 2 、IRC747-Fe 3+ and FPC22-Fe 3+ Each 1.0000g was put into a clean and dry 150mL Erlenmeyer flask, and 50mL of fluorine-containing waste...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com