Preparation method of nebivolol intermediate, intermediate for preparing nebivolol intermediate, and preparation method of intermediate
An intermediate and molar ratio technology, which is applied in the preparation of intermediate compound I and the intermediate compound I, and the preparation of nebivolol key intermediate compound II, can solve the problem of high pressure and corrosion resistance of equipment, Respond to the problems of high toxicity of raw materials and difficult purification of products, and achieve the effects of stable physical and chemical properties, convenient production and storage, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1 utilizes chromanic acid and 1,3-dichloropropane to prepare dichromanic acid ester 1a
[0062]
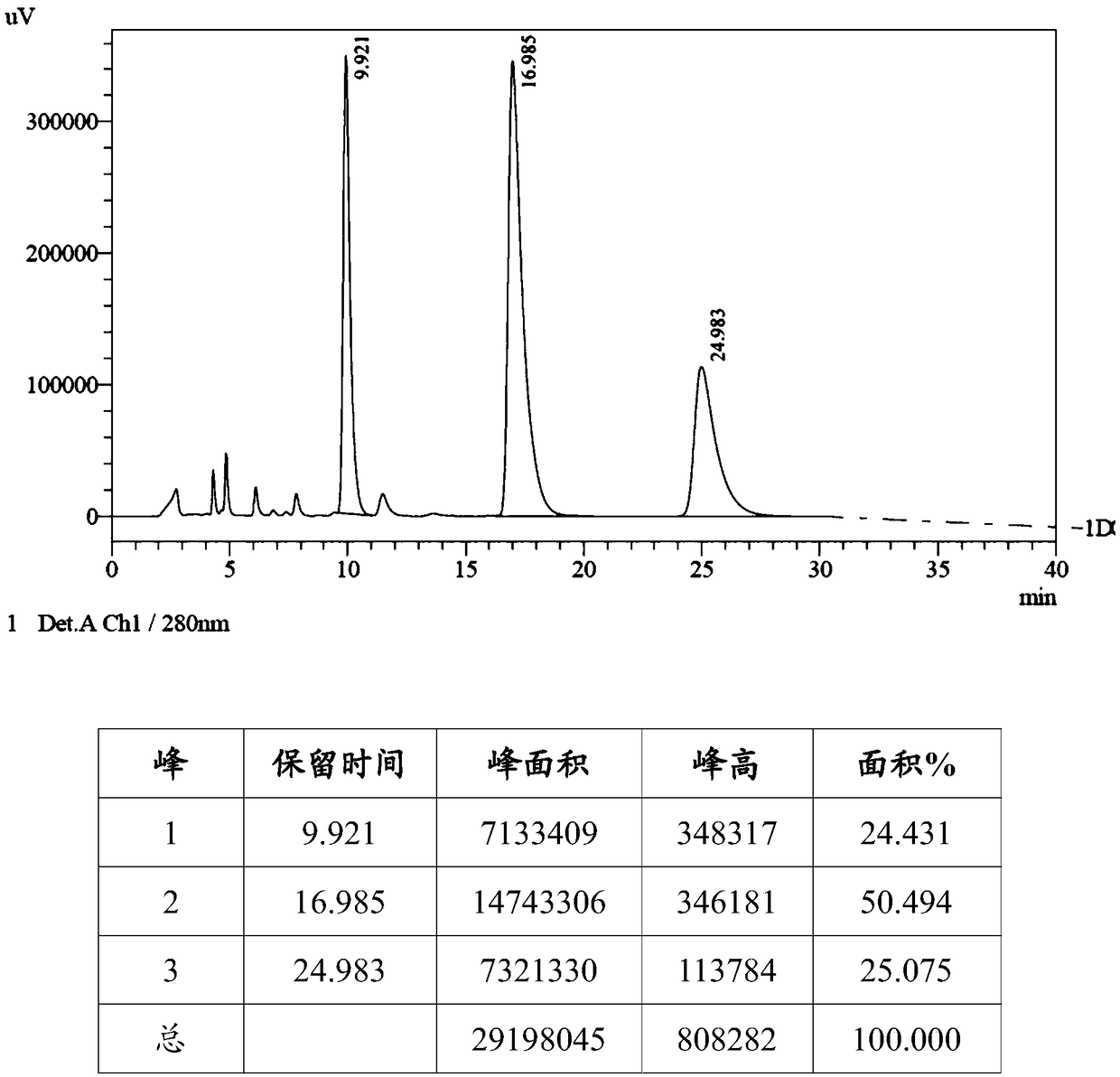
[0063] Mix 21.0g of chromanic acid (R configuration, 107mmol), 14.8g of potassium carbonate (107mmol), N,N-dimethylformamide (100mL) and toluene (40mL), then heat to 120°C, then 5.8 g 1,3-dichloropropane (51 mmol) was added to the reaction system, and the reaction was continued for 4 hours, and TLC detected that the reaction was complete. The reaction system was lowered to room temperature, acetic acid (2.2g) was added to quench the reaction, most of the solvent was distilled off under reduced pressure, water (100mL) was added, extracted three times with ethyl acetate (150mL), the combined organic phase was water (100mL ), the resulting organic phase was distilled under reduced pressure to viscous oil, recrystallized with isopropanol (40mL) to obtain 20.3g off-white solid, high performance liquid chromatography showed a single peak, the chromatographic purity ...
Embodiment 2
[0066] Embodiment 2 utilizes chromanic acid and 1,3-dichloropropane to prepare dichromanic acid ester 1a
[0067]
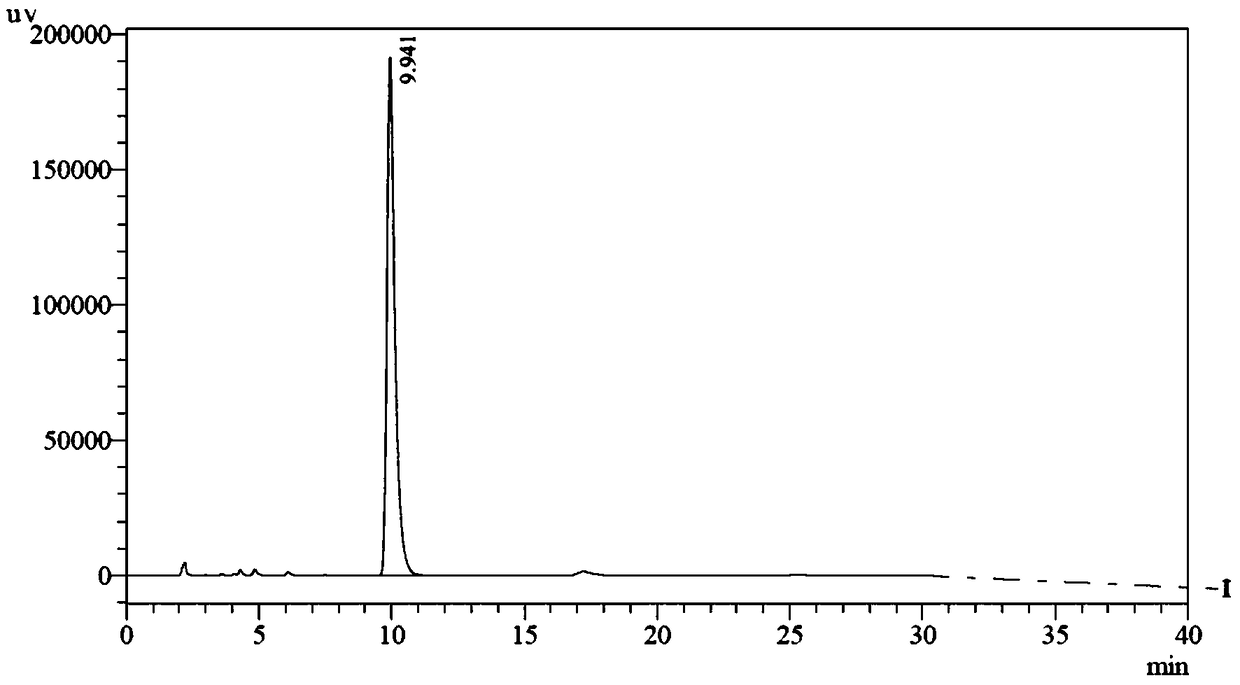
[0068] Mix 15.0g chromanic acid (R configuration, 76.5mmol), 12.0g potassium carbonate (87.0mmol), N,N-dimethylformamide (90mL) and toluene (30mL), then heat to 120°C, then 5.8 g of 1,3-dichloropropane (51 mmol) was added into the reaction system, and the reaction was continued for 4 hours, and the reaction was detected by TLC. The reaction system was lowered to room temperature, acetic acid (2.2g) was added to quench the reaction, most of the solvent was distilled off under reduced pressure, water (100mL) was added, extracted three times with ethyl acetate (150mL), the combined organic phase was water (100mL ), the resulting organic phase was distilled under reduced pressure to viscous oil, recrystallized with isopropanol (40mL) to obtain 10.2g off-white solid, high performance liquid chromatography showed a single peak, the chromatographic purity was 90%, an...
Embodiment 3
[0069] Embodiment 3 utilizes chromanic acid and 1,4-dichlorobutane to prepare dichromanic acid ester 1b
[0070]
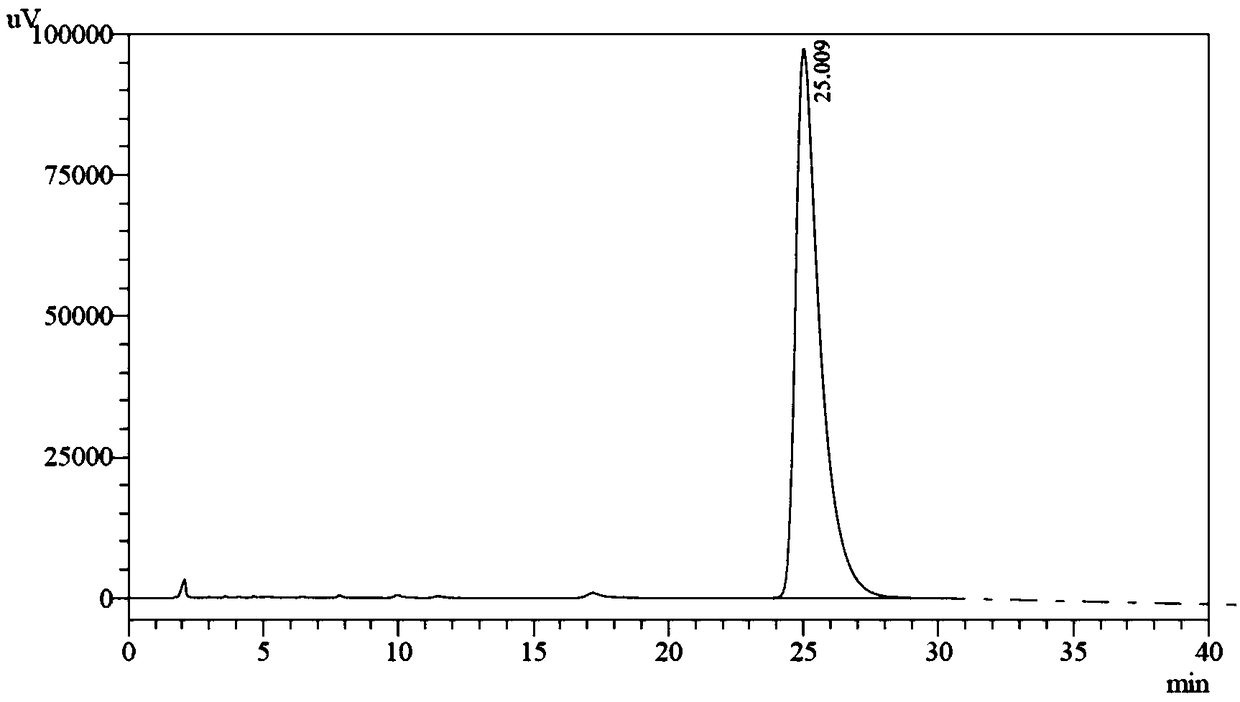
[0071] Add 2.9g chromanic acid (S configuration, 15mmol), 1.59g sodium carbonate (15mmol), N,N-dimethylformamide (15mL) into the reaction flask, and then add 0.63g 1,4-dichloro Butane (5 mmol) was added into the reaction system, heated to 120° C. for 5 hours, and the reaction was detected by HPLC. The reaction system was lowered to room temperature, acetic acid (0.3g) was added to quench the reaction, most of the solvent was distilled off under reduced pressure, water (15mL) was added, extracted three times with ethyl acetate (15mL), and the combined organic phases were extracted with water (15mL ) was washed, the resulting organic phase was distilled under reduced pressure to viscous oil, beating with isopropanol (10mL) to obtain 1.8g off-white solid, high performance liquid chromatography showed a single peak, the chromatographic purity was 95%, and the yield...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com