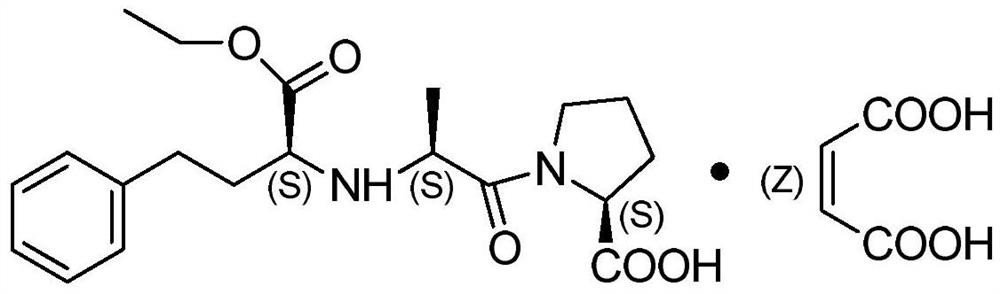
A kind of preparation method of enalapril maleate
A technology of enalapril maleate and enalapril, applied in the field of medicine, can solve the problems of unfavorable environment and personnel protection, unsuitable for industrialized production, cumbersome post-processing, etc., and achieve high yield and product purity, environmental protection Small pollution, improve the effect of labor protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Dissolve disodium hydrogen phosphate (20.4g, 2.0eq) in 100mL of water, add 160mL of dichloromethane after the system dissolves, N-[(S)-1-ethoxycarbonyl-3-phenylpropyl]-L- Alanine (20.0g, 1.0eq), stirred at room temperature. Dissolve triphosgene (8.4g, 0.4eq) in 40mL of dichloromethane, add dropwise to the reaction system at room temperature, add 0.5mL of pyridine after the reaction for 1h after the dropwise addition, stir for 1h and let stand for liquid separation, and concentrate the organic phase under reduced pressure to obtain an oily substance. Dissolve in 16mL of dichloromethane, add dropwise into 200mL of n-heptane, stir and crystallize at 0~10°C for 1h, filter under reduced pressure to obtain white solid N-[(S)-1-ethoxycarbonyl-3-phenylpropyl] -L-alanine-N-carboxylic acid anhydride, dried in vacuum at 50°C for 10h. 20.4 g was collected, and the yield was 91.6%.
[0047] Add L-proline (9.8g, 1.3eq), sodium hydroxide (3.4g, 1.3eq), 100mL water, and 70mL tetrahyd...
Embodiment 2
[0050] Dissolve disodium hydrogen phosphate (304.9g, 2.0eq) in 1.5L of water, add 900mL of dichloromethane after the system dissolves, N-[(S)-1-ethoxycarbonyl-3-phenylpropyl]-L - Alanine (300.0 g, 1.0 eq), stirred at room temperature. Dissolve triphosgene (127.5g, 0.4eq) in 600mL of dichloromethane, add it dropwise to the reaction system at room temperature, add 3mL of pyridine after 1h of reaction after the dropwise addition, stir for 1h and let it stand for liquid separation. The organic phase is concentrated under reduced pressure to obtain an oily substance, which is Dissolve 190mL of dichloromethane, add dropwise into 3L of n-heptane, stir and crystallize at 0-10°C, filter under reduced pressure to obtain a white solid, and vacuum-dry at 50°C for 10h. Receipt: 280.3g, yield 86.3%.
[0051] Add L-proline (131.4g, 1.3eq), sodium hydroxide (45.7g, 1.3eq), 1.3L water, and 0.9L tetrahydrofuran to dissolve at room temperature into a 2L three-necked flask. Cool down to 0-10°C ...
Embodiment 3
[0054] Dissolve disodium hydrogen phosphate (1.52kg, 2.0eq) in 7.5L of water, add 4.5L of dichloromethane after the system dissolves, N-[(S)-1-ethoxycarbonyl-3-phenylpropyl]- L-alanine (1.5kg, 1.0eq), stirred at room temperature. Dissolve triphosgene (0.64kg, 0.4eq) in 3L of dichloromethane, add dropwise to the reaction system at room temperature, add 15mL of pyridine after 1 hour of reaction after the dropwise addition, stir for 1 hour and let it stand for liquid separation. The organic phase is concentrated under reduced pressure to obtain an oily substance, which is Dissolve 0.6L of dichloromethane, add dropwise into 15.0L of n-heptane, stir and crystallize at 0-10°C, filter under reduced pressure to obtain a white solid, and vacuum-dry at 50°C for 10h. Receipt: 1.36kg, yield 82.9%.
[0055]Add L-proline (0.64kg, 1.3eq), sodium hydroxide (0.22kg, 1.3eq), 6.5L water, and 4.5L tetrahydrofuran into a 30L reactor and stir to dissolve at room temperature. Cool down to 0-10°C i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallization temperature | aaaaa | aaaaa |
| crystallization temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com