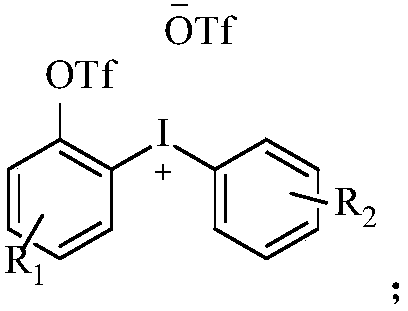
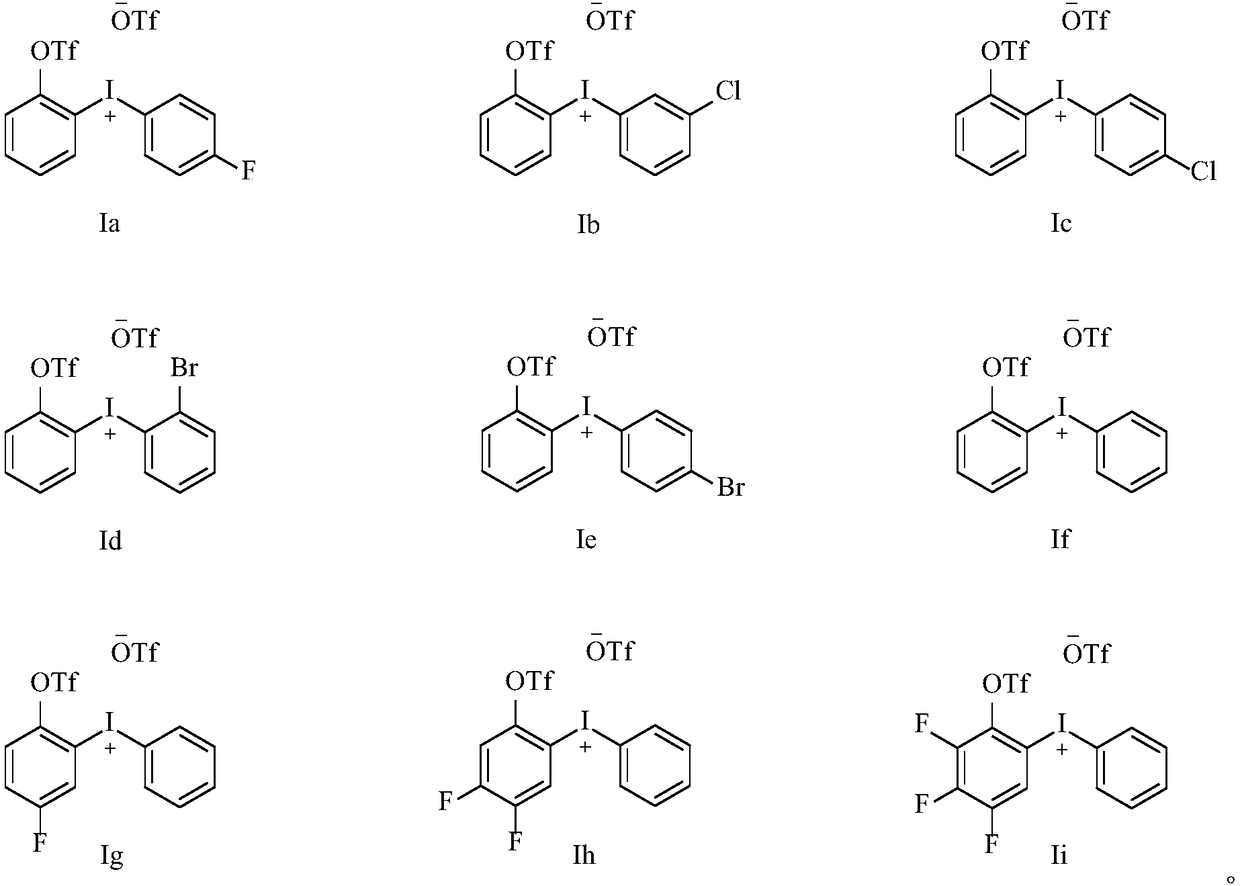
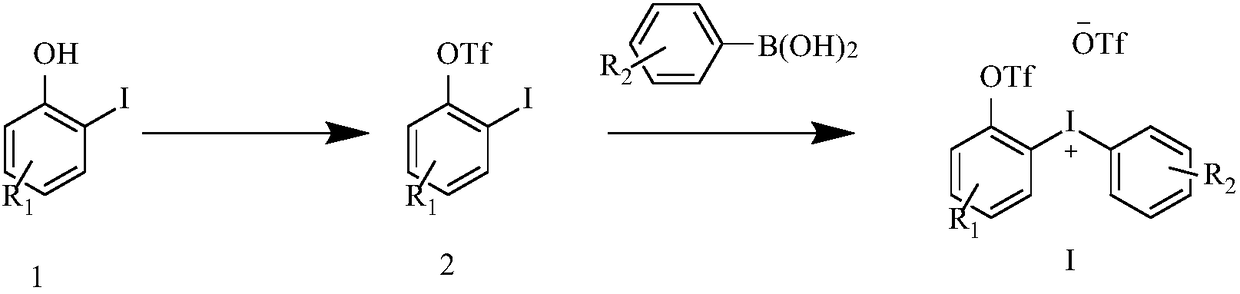
Diaryl iodide salt compound as well as preparation method and application thereof
A technology of diaryliodonium and compound, applied in the field of diaryliodonium salt compound and preparation thereof, can solve the problems of few research reports on the application of antibacterial agents, no preparation method and application of diaryliodonium salt compound are involved, and the like. The effect of high yield, simple synthesis, and antibacterial property promotion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation of diaryliodonium salt compound Ia:
[0037]
[0038] Place o-iodophenol (5mmol, 1equiv) in a 250mL round-bottomed flask, dissolve it in 100mL of dichloromethane (DCM), and add 1.25 equivalents of diisopropylethylamine and 1.25 equivalents of trifluoromethane in sequence at -78°C Sulfonic anhydride, reacted for 10 minutes and continued to react at room temperature for 2 hours to obtain compound 2a. Dissolve compound 2a in 40 mL of DCM, add 1.1 equivalents of m-chloroperoxybenzoic acid and 2.5 equivalents of boron trifluoride ether, react at room temperature for 1 hour, cool to 0°C, add 1.1 equivalents of 4-fluorophenylboronic acid and stir for 10 minutes, The reaction system was raised to room temperature and reacted for 30 minutes, 1.2 equivalents of trifluoromethanesulfonic acid was added at 0°C, raised to room temperature and reacted for 15 minutes, diaryliodonium salt compound Ia (80%) was obtained by recrystallization from ether.
[0039] Ia.M.p...
Embodiment 2
[0041] The preparation of diaryliodonium salt compound Ib:
[0042]
[0043]Place o-iodophenol (5mmol, 1equiv) in a 250mL round-bottomed flask, dissolve in 100mL DCM, add 1.25 equivalents of diisopropylethylamine and 1.25 equivalents of trifluoromethanesulfonic anhydride successively at -78°C, and react 10 After 2 minutes, the reaction was continued at room temperature for 2 hours to obtain compound 2a. Dissolve compound 2a in 40 mL of DCM, add 1.1 equivalents of m-chloroperoxybenzoic acid and 2.5 equivalents of boron trifluoride ether, react at room temperature for 1 hour, cool to 0°C, add 1.1 equivalents of 3-chlorophenylboronic acid and stir for 10 minutes, The reaction system was raised to room temperature and reacted for 30 minutes, 1.2 equivalents of trifluoromethanesulfonic acid was added at 0° C., raised to room temperature and reacted for 15 minutes, diaryliodonium salt compound Ib (70%) was obtained by diethyl ether recrystallization.
[0044] Ib. White solid, M....
Embodiment 3
[0046] Preparation of diaryliodonium salt compound Ic:
[0047]
[0048] Place o-iodophenol (5mmol, 1equiv) in a 250mL round-bottomed flask, dissolve in 100mL DCM, add 1.25 equivalents of diisopropylethylamine and 1.25 equivalents of trifluoromethanesulfonic anhydride successively at -78°C, and react 10 After 2 minutes, the reaction was continued at room temperature for 2 hours to obtain compound 2a. Dissolve compound 2a in 40 mL of DCM, add 1.1 equivalents of m-chloroperoxybenzoic acid and 2.5 equivalents of boron trifluoride ether, react at room temperature for 1 hour, cool to 0°C, add 1.1 equivalents of 4-chlorophenylboronic acid and stir for 10 minutes, The reaction system was raised to room temperature and reacted for 30 minutes, 1.2 equivalents of trifluoromethanesulfonic acid was added at 0° C., raised to room temperature and reacted for 15 minutes, diaryliodonium salt compound Ic (71%) was obtained by recrystallization from ether.
[0049] Ic. White solid, M.p.: 18...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com