Novel porphin diether salt compound as well as preparation method and application thereof
A technology of salt compounds and porphine bisethers, applied in the application of photodynamic therapy drugs, new porphine bisether salt compounds and the field of preparation thereof, can solve the problem that porphine bisether derivatives cannot be directly dissolved in water and the like , to achieve the effect of maintaining photodynamic activity, a single component, and eliminating potential adverse effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
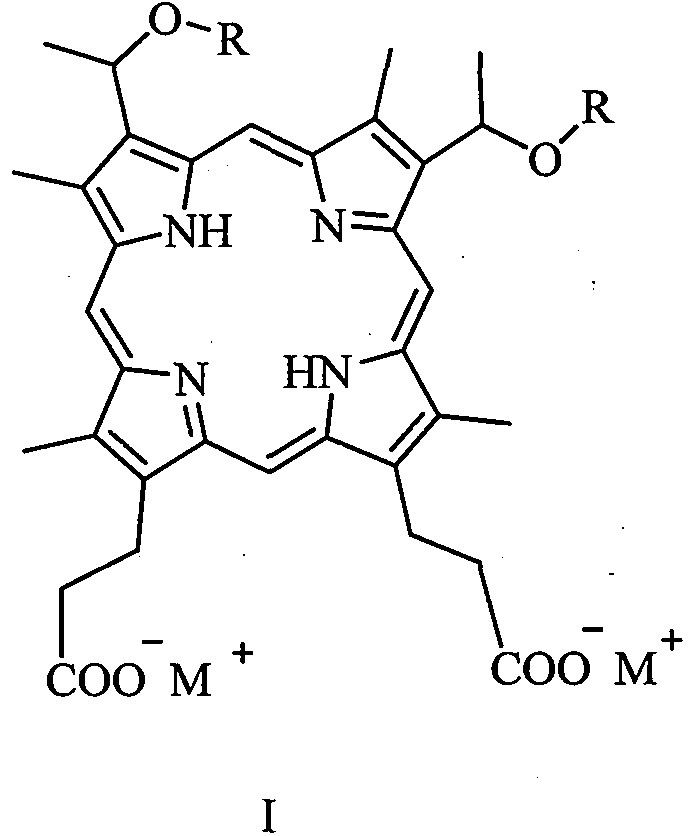
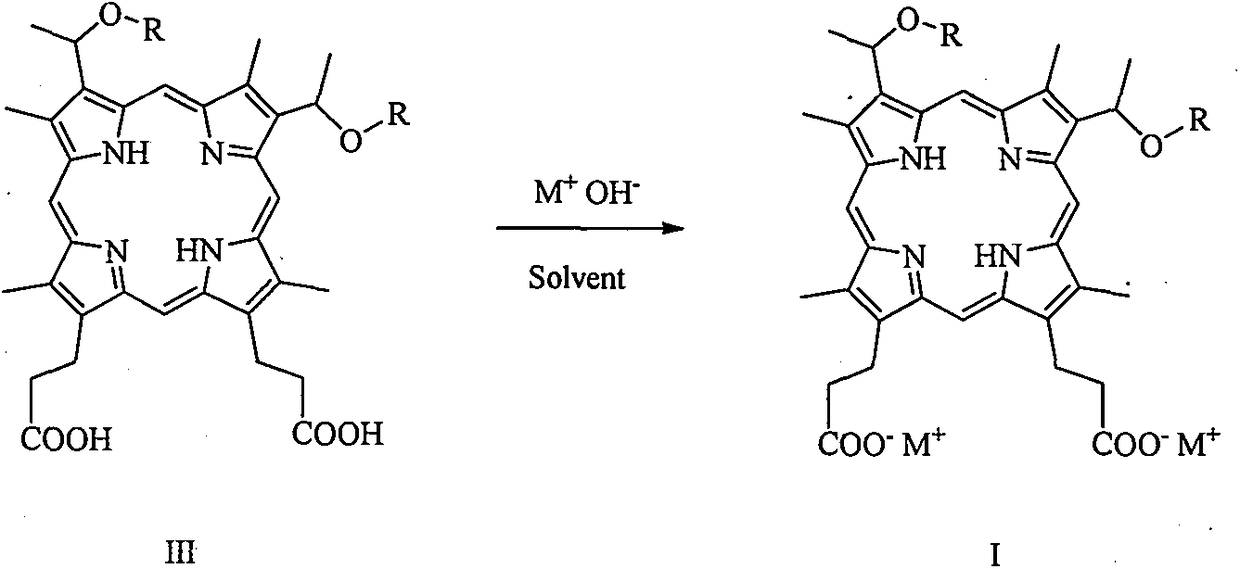
[0042] 3, the preparation of 8-bis (1-methoxyethyl) hypoporphyrin IX disodium salt (abbreviated as porphine dimethyl ether disodium salt):
[0043] Add 10 g (16.0 mmol) of 3,8-bis(1-methoxyethyl) hypoporphyrin IX (porphine dimethyl ether for short) into a 100 mL flask, add 60 mL of ethanol, and stir to dissolve the solid. 16.0 mL (32.0 mmol) of 2 mol / L aqueous sodium hydroxide solution was slowly added, and stirring was continued for 30 minutes. The solvent was removed under reduced pressure and dried in vacuo to obtain 10.7 g of 3,8-bis(1-methoxyethyl) hypoporphyrin IX disodium salt with a yield of about 100%. MS (ESI) m / z: 627.2 [M-2Na+1] + , 648.2 [M-Na] + , 670.3[M] + . Elemental analysis: C, 64.47; H, 5.97; N, 8.35; O, 14.31; Na, 6.86. Flame reaction is yellow.
[0044]
Embodiment 2
[0046] 3, the preparation of 8-bis (1-n-propoxyethyl) hypoporphyrin IX disodium salt (abbreviated as porphine dipropyl ether disodium salt)
[0047] Add 10.9 g (16.0 mmol) of 3,8-bis(1-n-propoxyethyl) hypoporphyrin IX (abbreviated as porphin dipropyl ether) into a 100 mL flask, add 60 mL of ethanol, and stir to dissolve the solid. 16.0 mL (32.0 mmol) of 2 mol / L aqueous sodium hydroxide solution was slowly added, and stirring was continued for 30 minutes. The solvent was removed under reduced pressure and dried in vacuo to obtain 11.6 g of 3,8-bis(1-n-propoxyethyl) hypoporphyrin IX disodium salt with a yield of about 100%. MS (ESI) m / z: 682.2 [M-2Na+1] + , 704.2 [M-Na] + , 726.3[M] + . Elemental analysis: C, 66.10; H, 6.66; N, 7.71; O, 13.21; Na, 6.33. Flame reaction is yellow.
[0048]
Embodiment 3
[0050] Preparation of 3,8-two (1-n-butoxyethyl) hypoporphyrin IX disodium salt (abbreviated as porphine dibutyl ether disodium salt)
[0051] Add 11.4 g (16.0 mmol) of 3,8-bis(1-n-butoxyethyl) hypoporphyrin IX (abbreviated as porphine bis-butyl ether) into a 100 mL flask, add 60 mL of ethanol, and stir to dissolve the solid. 16.0 mL (32.0 mmol) of 2 mol / L aqueous sodium hydroxide solution was slowly added, and stirring was continued for 30 minutes. The solvent was removed under reduced pressure and dried in vacuo to obtain 12.1 g of 3,8-bis(1-n-butoxyethyl) hypoporphyrin IX disodium salt with a yield of about 100%. MS (ESI) m / z: 710.4 [M-2Na + +1] + , 732.3[M-Na] + , 754.4[M] + . Elemental analysis: C, 66.83; H, 6.94; N, 7.42; O, 12.72; Na, 6.09. Flame reaction is yellow.
[0052]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com