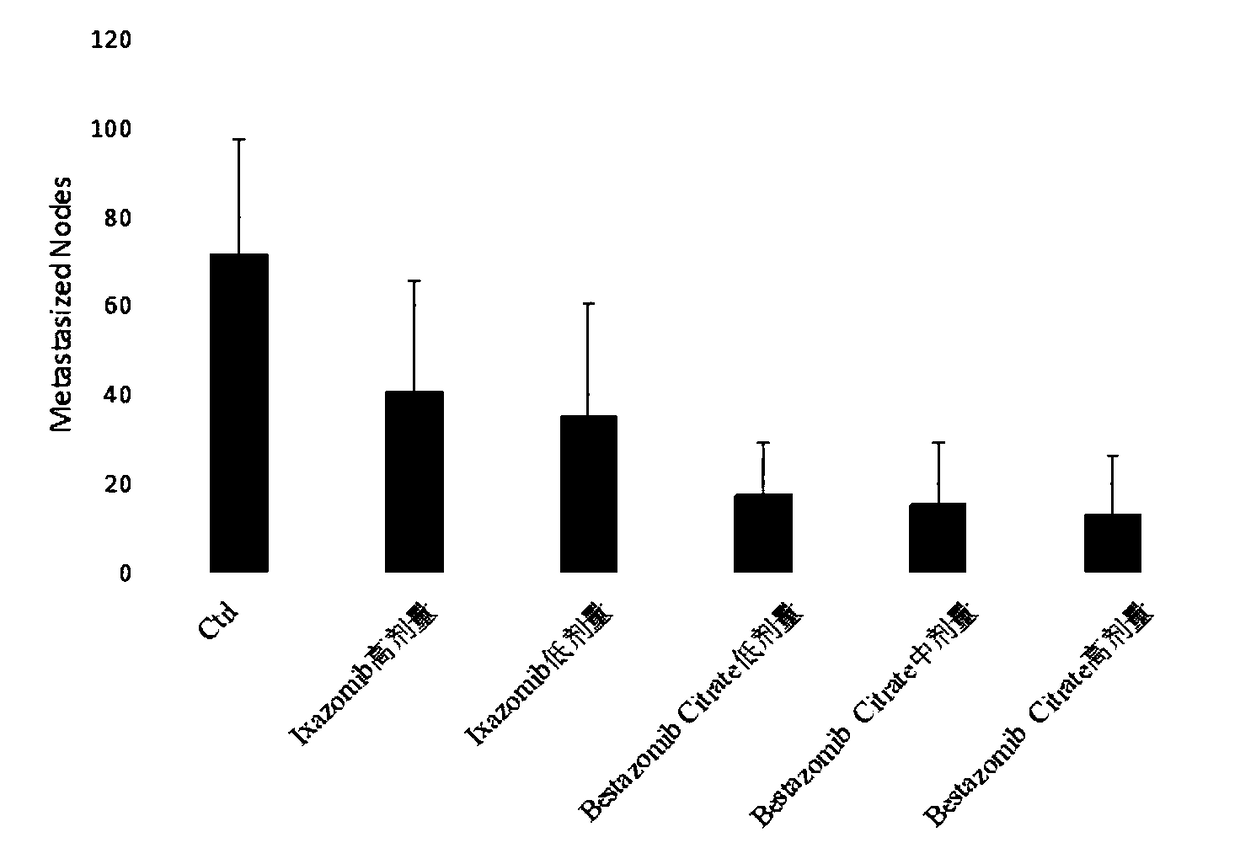
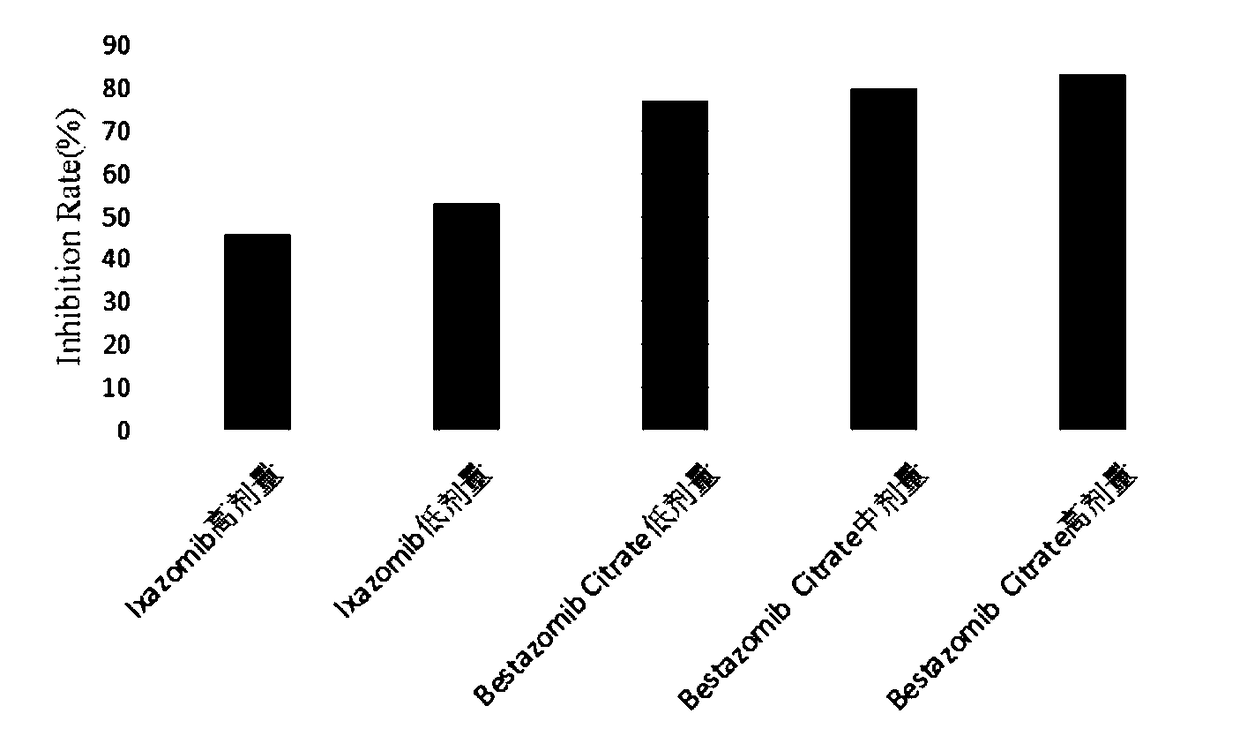
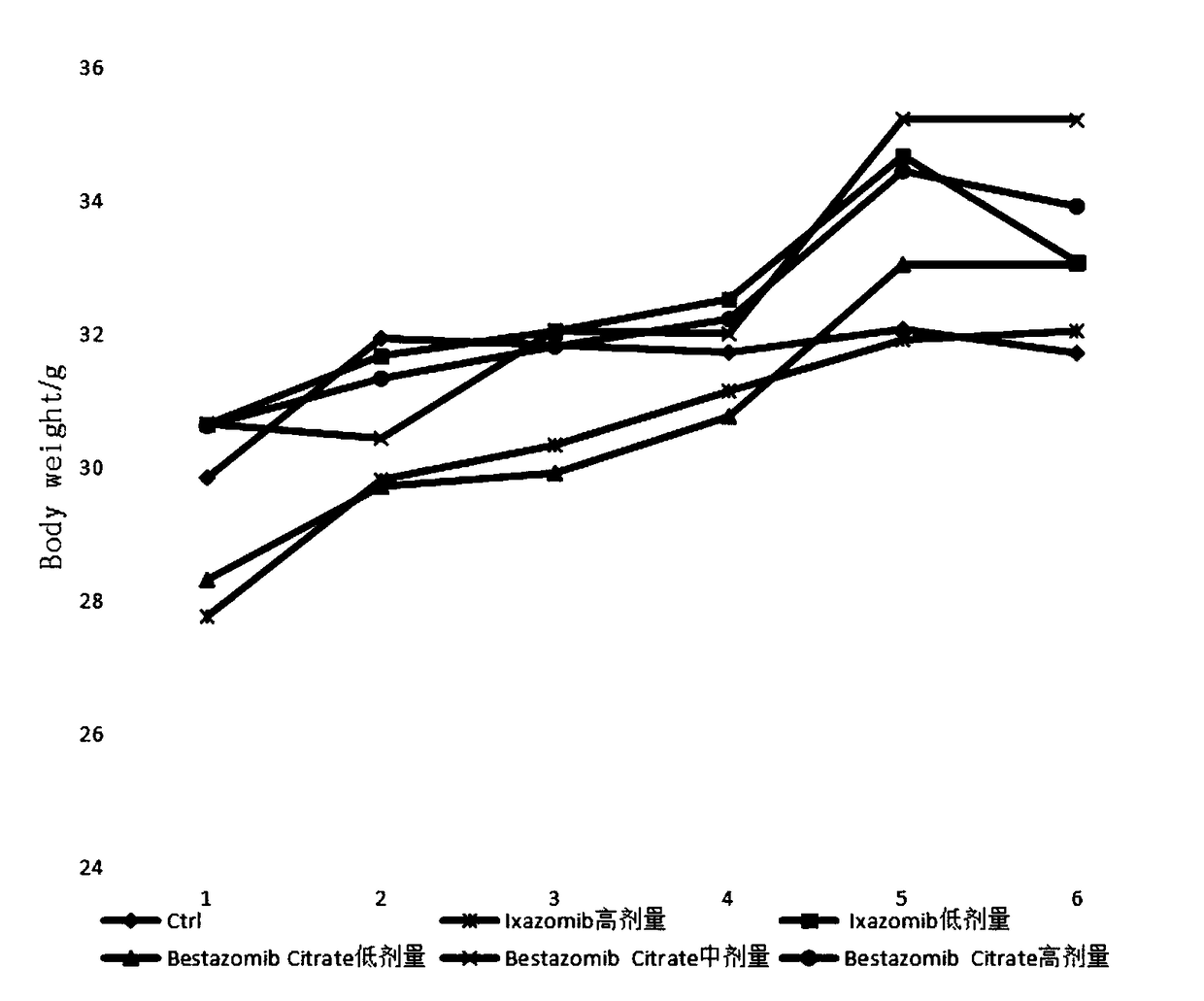
Multifunctional immunity-targeted micromolecule anti-cancer medicine (Bestazomib citrate) and preparation method and application thereof
A pharmacy and drug technology, applied in anti-tumor drugs, chemical instruments and methods, drug combinations, etc., can solve problems such as increasing treatment costs, and achieve the effect of reducing treatment costs and avoiding dangers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1: (2S,3R)-3-((Benzyloxycarbyl)amino)-2-hydroxy-4-phenylbutyric acid (2)
[0068] AHPA (1, 1.95g, 10.0mmol) was dissolved in a mixed solution of 100mL tetrahydrofuran and 1mol / L sodium hydroxide, and benzyloxycarbonyl chloride (1.88g, 11.0mmol) was added dropwise under ice-cooling conditions. After reacting at room temperature for 6 hours, the tetrahydrofuran in the reaction solution was distilled off, the aqueous phase was adjusted to pH=1 with 1 mol / L hydrochloric acid, extracted three times with ethyl acetate, the organic phases were combined, dried over anhydrous magnesium sulfate, and evaporated to dryness to obtain a white solid 2( 2.11 g, 64%).
Embodiment 2
[0069] Example 2: (R)-1-((2S,3R)-3-((Benzyloxycarbonyl)amino)-2-hydroxyl-4-phenylbutyrylamino)-3-methylbutylboronic acid pinanedi Alcohol Esters (3)
[0070] Compound 2 (3.29g, 10.0mmol) was dissolved in 50mL of anhydrous DCM, HOBt (1.49g, 11mmol) and EDCI (2.10g, 11.0mmol) were added under ice-bath conditions, after 0.5h, (R)-1- Amino-3-methylbutyl borate pinanediol trifluoroacetate (4.07g, 11.0mmol), triethylamine 1.5mL. Remove the ice bath and react at room temperature for 5h. After the reaction was completed, the organic layer was washed with water, 1M citric acid, saturated sodium bicarbonate, and saturated sodium chloride, respectively. Dry over anhydrous magnesium sulfate, filter, and evaporate the solvent to give 3 (3.05 g, 53%) as a white solid. 1 H-NMR (400MHz DMSO-d6):0.86-0.88(d,3H),0.90-0.92(d,3H),1.03-1.06(s,3H),1.11-1.17(m,4H),1.38-1.53( m,8H),1.76-1.82(m,1H),2.05-2.10(m,1H),2.26-2.31(m,1H),2.78-2.82(m,1H),2.96-3.00(m,1H), 3.42-3.45(t,1H),3.61-3.63(t,1H),4....
Embodiment 3
[0071] Example 3: (R)-1-((2S,3R)-3-((benzyloxycarbonyl)amino)-2-hydroxy-4-phenylbutyrylamino)-3-methylbutylboronic acid (4)
[0072] Compound 3 (2.88g, 5.0mmol) was dissolved in 50mL of anhydrous methanol and n-hexane 1:1 mixed solvent, and isobutylboronic acid (1.27g, 12.5mmol) was added thereto. Under the condition of ice bath, add 1mol / L hydrochloric acid solution and continue to stir for 6h at room temperature. After static separation, the lower layer was dissolved by adding 2mol / L sodium hydroxide, and extracted three times with dichloromethane. The aqueous phase was adjusted to pH=5 with 1N hydrochloric acid, extracted three times with DCM, combined with DCM phases, washed with saturated brine, dried over anhydrous magnesium sulfate, and evaporated to dryness to obtain white solid 4 (0.77 g, 35%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com