Mutant Bordetella strains and methods of use thereof
A bacillus, subject technology, applied in the fields of immunology, microbiology, allergy and medicine, which can solve the problems of poor immunogenicity, troublesome attenuation, difficult to produce, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1- 100
[0065] Example 1 - Construction and characterization of a pertactin-deficient strain of Bordetella pertussis.
[0066] Escherichia coli DH5α, SM10 and Bordetella pertussis BPZE1, BPSM (Menozzi et al., Infect Immun [Infection and Immunity] 1994; 62:769-778) and B1917 (Bart et al. Bulletin] 2014; 2(6)). Bordetella strains were grown at 37°C on Bordet-Gengou agar (BG) supplemented with 1% glycerol and 10% defibrinated sheep blood. After growth, bacteria were harvested by scraping the plates and resuspended in phosphate-buffered saline (PBS) at the desired density. For liquid culture, Bordetella strains were incubated at 37°C in a modified Stainer- Grow in Scholte's medium (Imaizumi et al. Infect Immun [Infection and Immunity] 1983; 41:1138-1143). E. coli strains used in the cloning procedure were grown in LB broth or LB agar plates. Streptomycin (Sm) was used at 100 μg / ml, gentamicin (Gm) at 10 μg / ml, and ampicillin (Amp) at 100 μg / ml as needed.
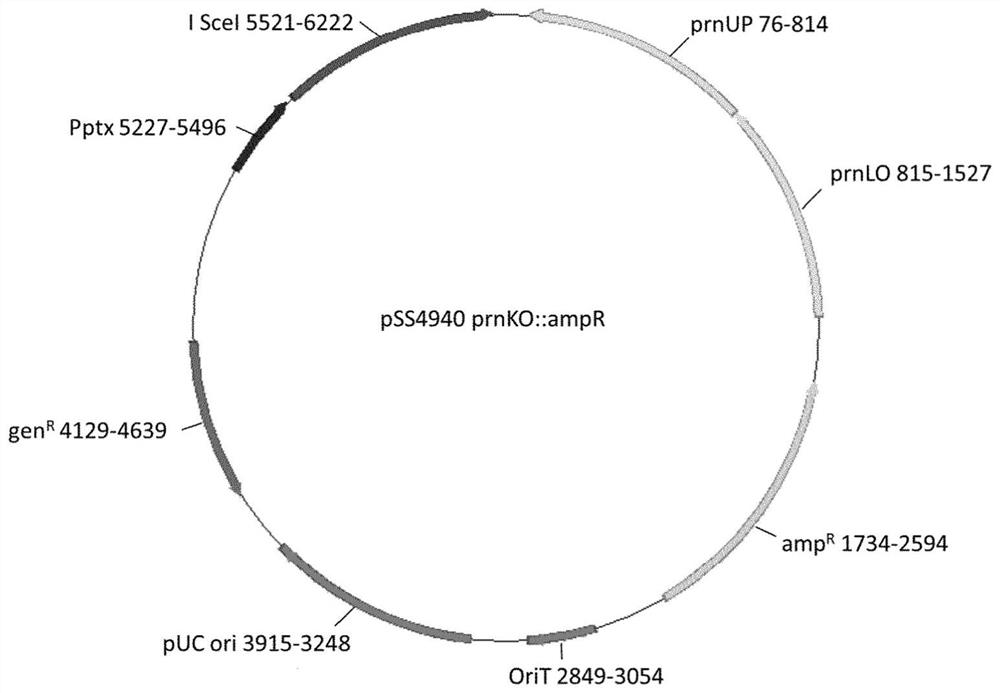
[0067] To delete the gene p...
example 6
[0078] Example 6 - Measurement of lung cell infiltration and cytokine profiles.
[0079] Evaluate Figure 6 Infiltration of airway cell populations in allergic mice from the experiments shown in and discussed immediately above. Following plethysmography measurements, bronchoalveolar lavage (BAL) fluid was collected to measure cellular infiltration in the airways. Cells from BAL fluid were harvested by centrifugation at 1,200 rpm for 5 minutes at 4°C, resuspended in PBS for cell counting using Shandon cytospin 4 (Thermo Fisher Scientific), and analyzed with May Grünwald Giemsa Staining is used for cell type counts. The total cell number was measured in the BAL fluid of mice ( Figure 7A ), and count eosinophils ( Figure 7B ), neutrophils ( Figure 7C ), lymphocytes ( Figure 7D ) and macrophages ( Figure 7E ) percentage. Statistical analysis was performed by applying a parametric one-way ANOVA test using a post-hoc Bonferroni comparison test (95% confidence interval)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com