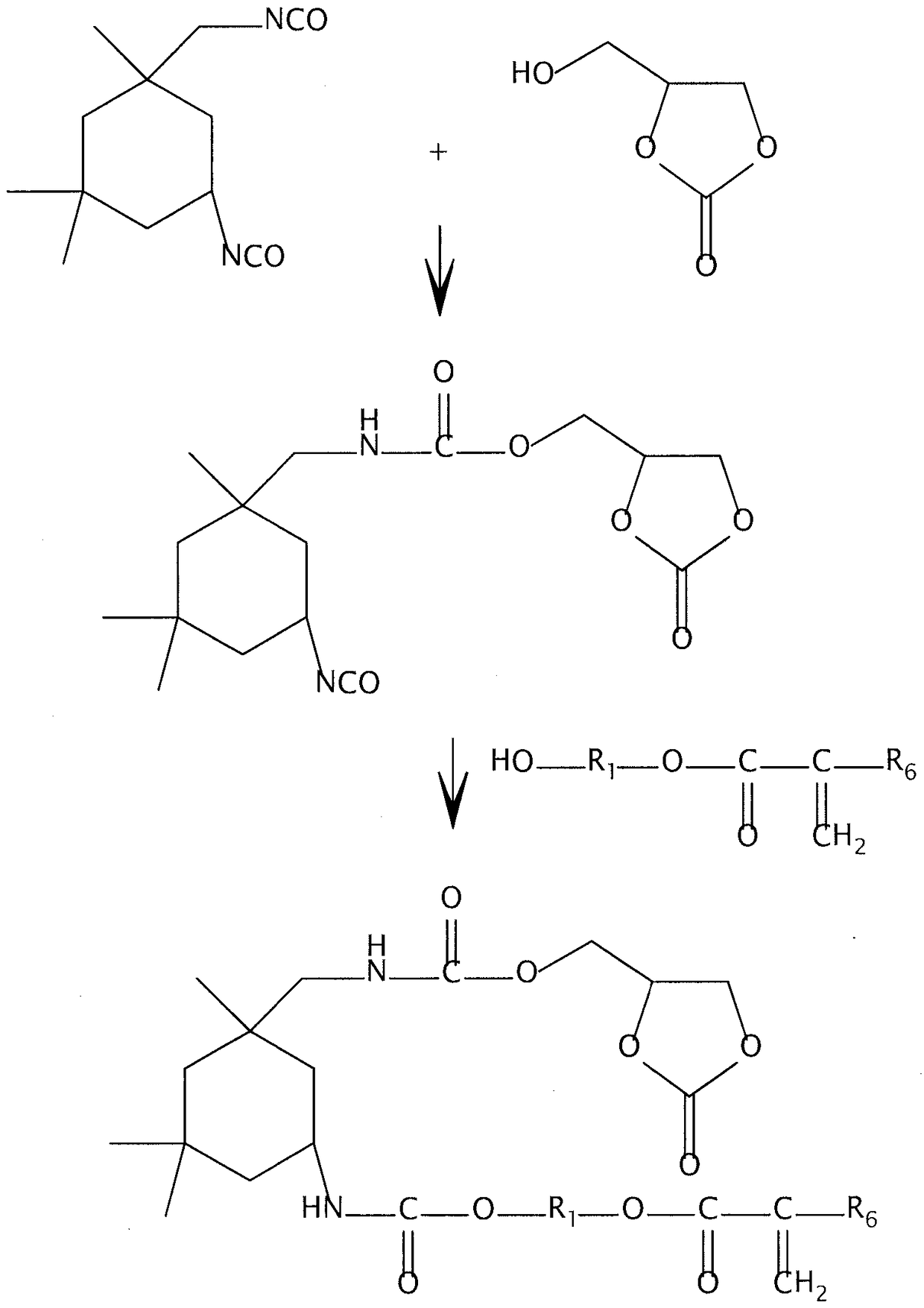
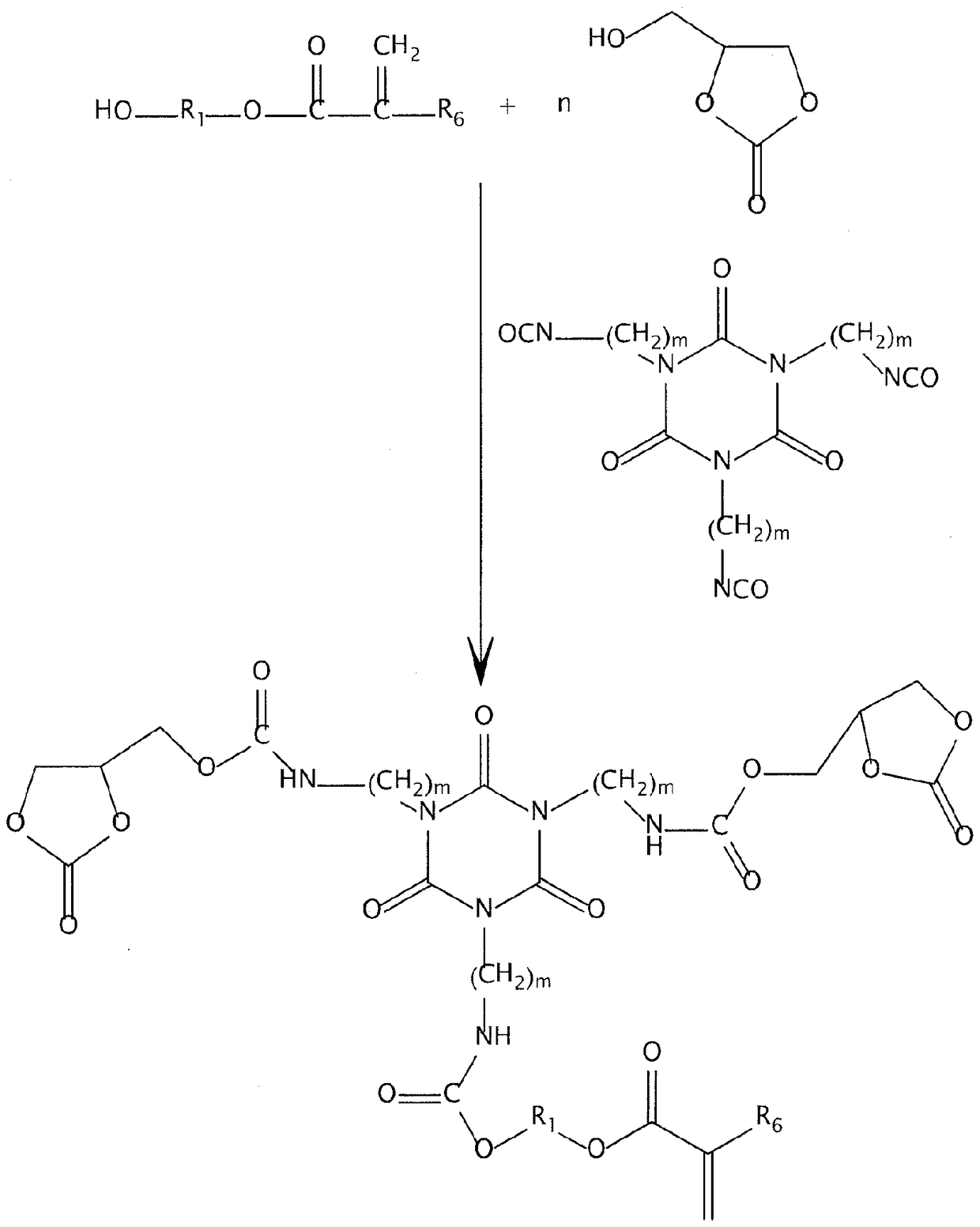
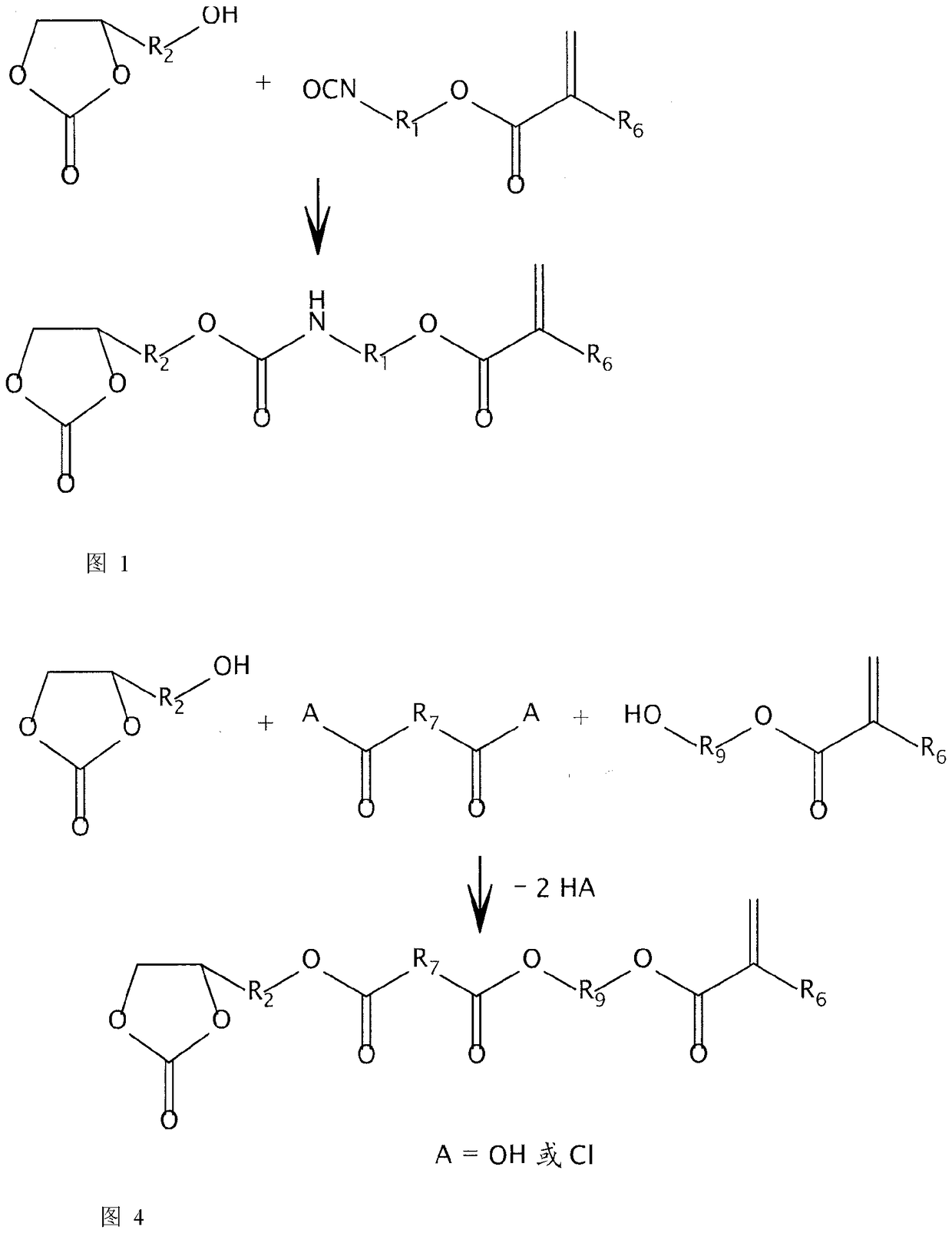
Non-isocyanate polyurethane inks for 3D printing
An ink and three-dimensional printing technology, applied in polyurea/polyurethane coatings, inks, 3D object support structures, etc., can solve problems such as toxicity, and achieve the effects of reducing toxicity, reducing skin sensitization or irritation, and high toughness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0159] Cyclic carbonate monomer
[0160] The cyclic carbonate monomer with formula (A11) structure is prepared as follows, wherein R 1 for (CH 2 ) 8 . To a 500 mL 3-neck round bottom flask with a large Teflon®-coated football-shaped stirring magnet, a constant pressure addition funnel, and a condenser, was charged 12.0 g of sebacoyl chloride (SebCl), 10.4 g of triethylamine, and 80 g of methyltetrahydrofuran (mTHF ) solvent. Place this flask into a 190x 100 Pyrex filled with ice water ® (2.5L) crystallization dish and allow to stir / cool for about 30 min. 11.8 grams of glycerol carbonate and 20 grams of mTHF were added to a 150 mL constant pressure addition funnel, followed by gentle vortexing until the solid glycerol carbonate dissolved. The glycerol carbonate solution was added dropwise to the cooled sebacoyl chloride solution over a 30 minute period. Additional mTHF was added during subsequent reactions to facilitate stirring. Stirring was continued for about two ...
Embodiment 2
[0162] Cyclic carbonate monomer
[0163] A cyclic carbonate monomer having a structure of formula (A14) is prepared as follows, wherein R 1 for (CH 2 ) 2 , R 2 for CH 2 , and R 6 for CH 3 or H. for R 6 =CH 3 , was added 620.6 grams of isocyanatoethyl methacrylate (available from Synasia, Nantong, China) to a 2000 mL beaker with a large football-shaped stirring magnet coated with Teflon®. The solution was then stirred and heated in a 60°C silicone oil bath. A mixture of 476.7 grams of glycerol carbonate (from Huntsman Chemical) and 0.16 grams of dibutyltin dilaurate (from Sigmal Aldrich) was gradually added to the heated isocyanatoethyl methacrylate while maintaining the reaction temperature at 50 °C and between 80°C. After all the mixture had been added, 0.48 grams of BHT (from Sigma-Aldrich) was added and the resulting solution was stirred at 70-80°C for an additional 16 hours. The resulting product was a clear viscous liquid with no isocyanate peaks by FT-IR. ...
Embodiment 3
[0165] Cyclic carbonate monomer
[0166] The cyclic carbonate monomer having the structure of formula (A15) is prepared as follows. To a 500 mL beaker with a large football-shaped stirring magnet coated with Teflon® was added 55.57 grams of isophorone diisocyanate (from Sima-Aldrich), isobornyl methacrylate (IBOMA, from Evonik Industries) and 0.02 grams of diisocyanate (available from Evonik Industries). Dibutyltin laurate (available from Sigma Aldrich). The solution was stirred and heated in a silicone oil bath at 55-60°C. In the next step, 29.5 grams of glycerol carbonate (from Huntsman Chemical) was gradually added over 30 min while maintaining the reaction temperature between 50°C and 80°C. After all the glycerol carbonate had been added, 0.04 grams of BHT (from Sigma-Aldrich) was added and the resulting solution was stirred at 60-70°C for an additional hour. Then, 32.53 grams of hydroxyethyl methacrylate (from Sigma-Aldrich) were gradually added over 0.2 hours, and t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com