Preparation process of hematodialysis concentrate A powder
A technology of hemodialysis and preparation process, applied in the field of hemodialysis, can solve the problems of unstable product quality of hemodialysis concentrate A powder, increase in transportation and storage costs, and increase in dissolution time, and achieve easy preparation and storage, and reduce product differences. Small, stable product quality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
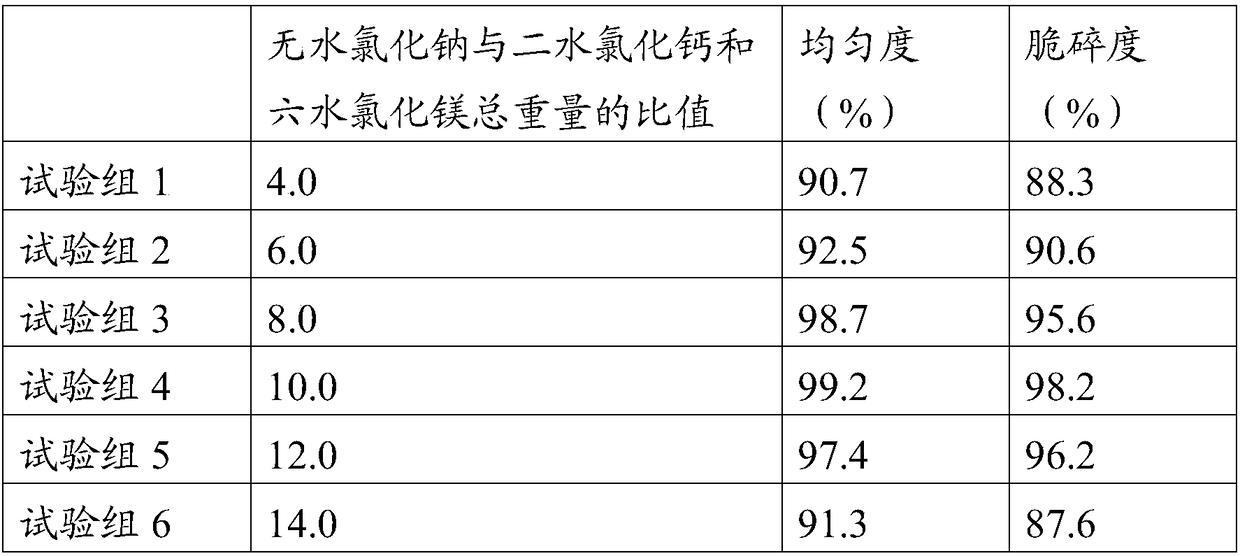
[0038] A hemodialysis concentrate A powder, comprising the following components by weight: 16000 g of anhydrous sodium chloride, 500 g of anhydrous potassium chloride, 600 g of calcium chloride dihydrate, 300 g of magnesium chloride hexahydrate and 600 g of anhydrous citric acid.
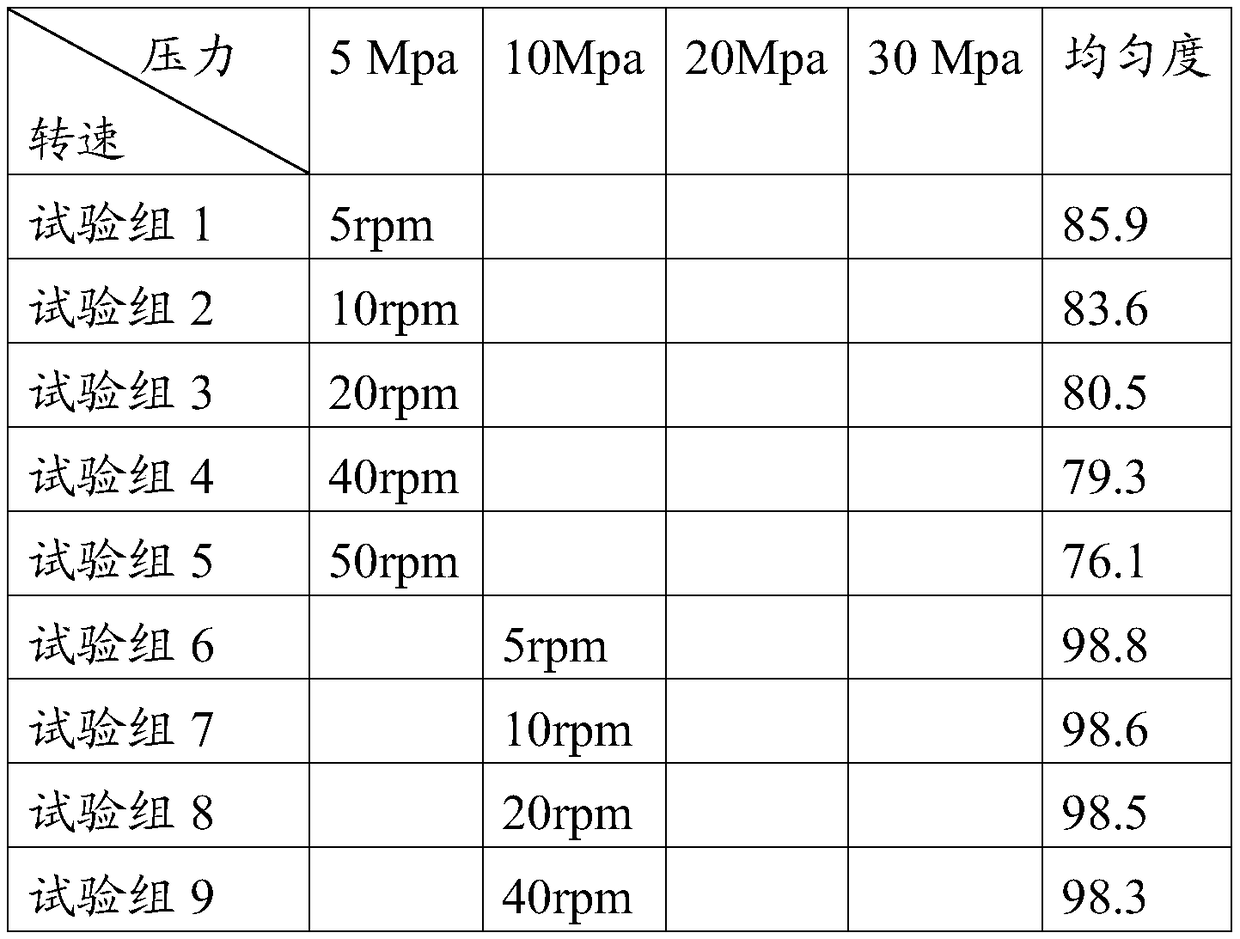
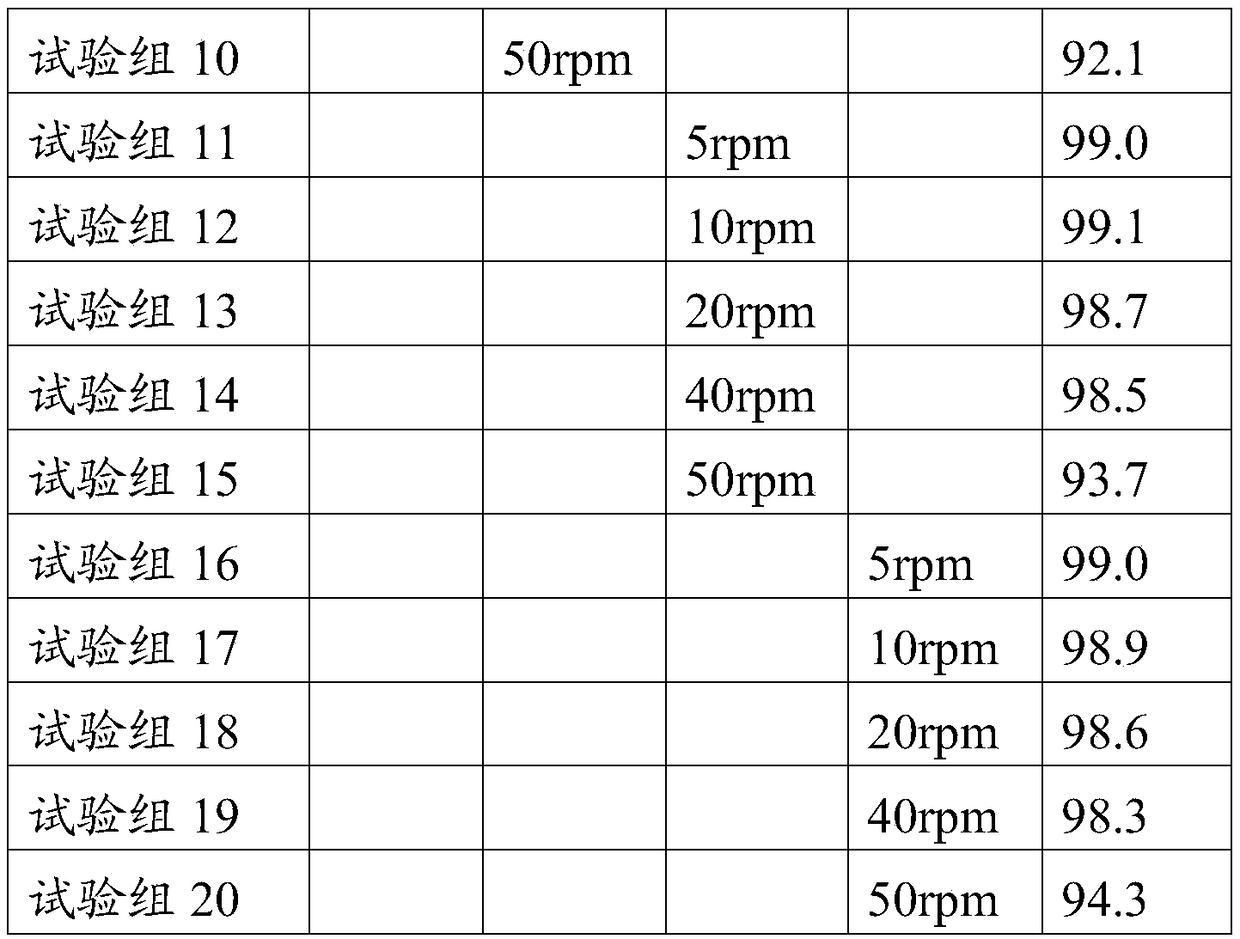
[0039] A preparation process of hemodialysis concentrate A powder, the operation process is completed in the operation room, and the temperature of the operation room is adjusted to 15°C, relative humidity ≤ 40%, and planktonic bacteria ≤ 100 / m 3 , sedimentation bacteria ≤ 3 / petri dish, and the pressure difference between adjacent operations ≥ 10Pa, specifically include the following steps:
[0040] (1) Take each raw material according to the above-mentioned a kind of hemodialysis concentrate A powder, and set aside;
[0041](2) Add calcium chloride and magnesium chloride to the mixing tank of the three-dimensional mixer through the feeding port after sieving, and then add anhydrous sodium chloride ...
Embodiment 2
[0047] A hemodialysis concentrate A powder, comprising the following components by weight: 22000g of anhydrous sodium chloride, 800g of anhydrous potassium chloride, 900g of calcium chloride dihydrate, 400g of magnesium chloride hexahydrate, 1000g of anhydrous citric acid and anhydrous Water glucose 7000g.
[0048] A preparation process of hemodialysis concentrate A powder, the operation process is completed in the operation room, the temperature of the operation room is adjusted to 30°C, the relative humidity is ≤40%, and the planktonic bacteria is ≤100 / m 3 , sedimentation bacteria ≤ 3 / petri dish, and the pressure difference between adjacent operations ≥ 10Pa, specifically include the following steps:
[0049] (1) Take each raw material according to the above-mentioned a kind of hemodialysis concentrate A powder, and set aside;
[0050] (2) Add calcium chloride and magnesium chloride to the mixing tank of the three-dimensional mixer through the feeding port after sieving, an...
Embodiment 3
[0056] A hemodialysis concentrate A powder, comprising the following components by weight: 21070g of anhydrous sodium chloride, 522g of anhydrous potassium chloride, 643g of calcium chloride dihydrate, 356g of magnesium chloride hexahydrate, 739g of anhydrous citric acid and anhydrous Water glucose 2000g.
[0057] A preparation process of hemodialysis concentrate A powder, the operation process is completed in the operation room, and the temperature of the operation room is adjusted to 20°C, relative humidity ≤ 40%, and planktonic bacteria ≤ 100 / m 3 , sedimentation bacteria ≤ 3 / petri dish, and the pressure difference between adjacent operations ≥ 10Pa, specifically include the following steps:
[0058] (1) Take each raw material according to the above-mentioned a kind of hemodialysis concentrate A powder, and set aside;
[0059] (2) Add calcium chloride and magnesium chloride to the mixing tank of the three-dimensional mixer through the feed port after sieving, and then add a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com