Single-ion conductor polymer lithium salt and preparation method thereof
A conductor polymer, single-ion technology, which is used in the preparation of bisbenzenesulfonimide lithium-based single-ion polymer lithium salt, and the application field of polymer electrolytes, which can solve the problem of low ionic conductivity and lithium ion migration number. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
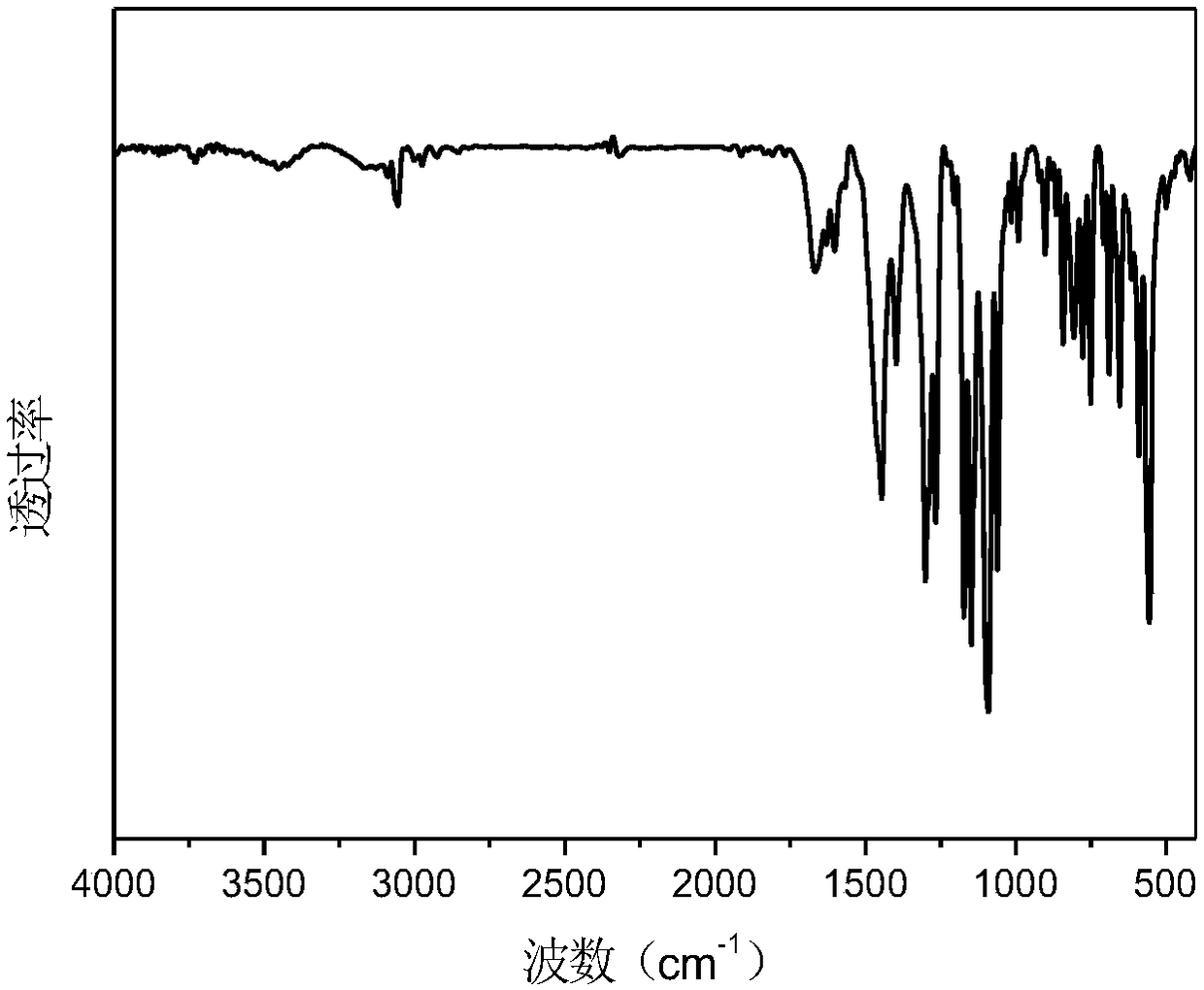
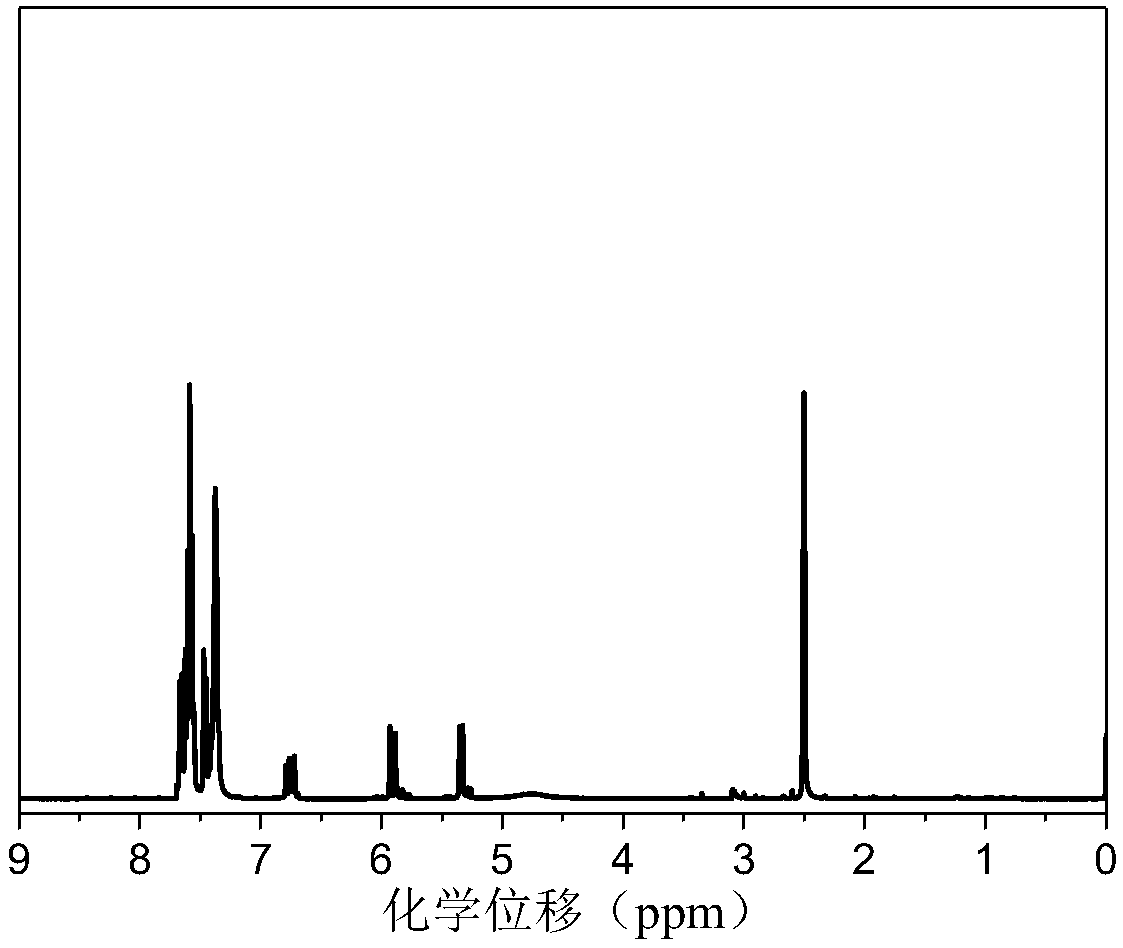
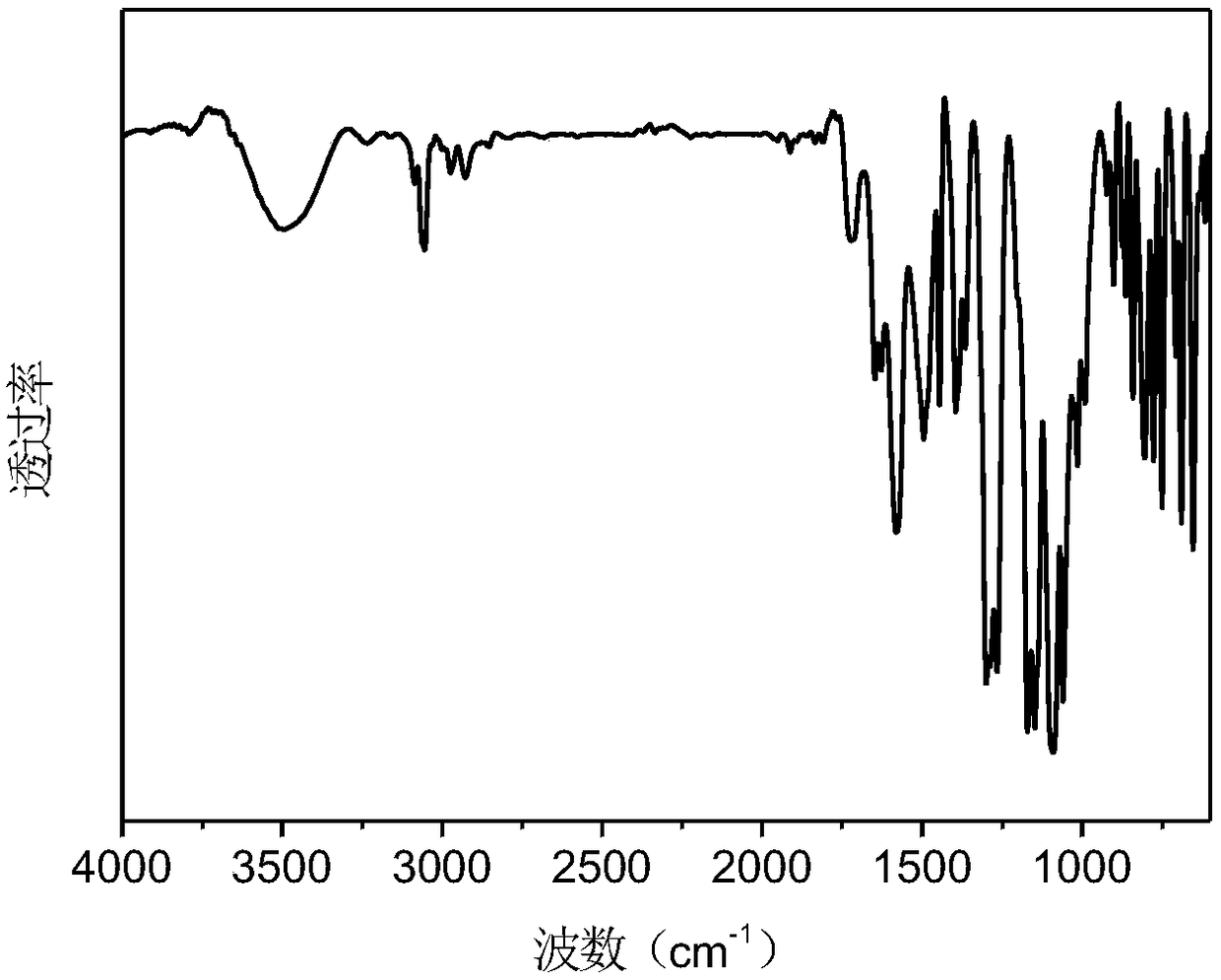
Image
Examples
Embodiment 1
[0032] The preparation of embodiment 1-styryl bissulfonylimide lithium salt and maleic anhydride alternating polymer P (STSSILi-alt-MA)
[0033]Pour the lithium salt monomer STSSILi and maleic anhydride (MA) into the polymerization tube according to the molar ratio of 1:1.2, add dimethyl sulfoxide (DMSO) to dissolve, add 0.1% (molar ratio) of azobisisobutyl Nitrile (AIBN). Put the polymerization tube into liquid nitrogen to cool it, carry out nitrogen gas-evacuation three times, seal the tube with an alcohol torch and put it in an oil bath at 60°C for 48 hours to react. After the reaction, the polymerization tube was opened to expose to the air. The DMSO solution of the polymer was precipitated three times in tetrahydrofuran to remove unreacted monomer.
Embodiment 2
[0034] The preparation of embodiment 2-styryl bissulfonylimide lithium salt and maleic anhydride alternating polymer P (STSSILi-alt-MA)
[0035] Pour the lithium salt monomer STSSILi and maleic anhydride (MA) into the polymerization tube according to the molar ratio of 1:1.05, add dimethyl sulfoxide (DMSO) to dissolve, add 0.2% (molar ratio) of azobisisobutyl Nitrile (AIBN). Put the polymerization tube into liquid nitrogen to cool it, carry out nitrogen gas-evacuation three times, seal the tube with an alcohol torch and put it in an oil bath at 80°C for 36 hours to react. After the reaction, the polymerization tube was opened to expose to the air. The DMSO solution of the polymer was precipitated three times in tetrahydrofuran to remove unreacted monomer.
Embodiment 3
[0036] Example 3 - Preparation of styryl bissulfonylimide lithium salt and maleic anhydride alternating polymer P (STSSILi-alt-MA) Lithium salt monomer STSSILi and maleic anhydride (MA) in a molar ratio of 1:1.2 Pour into a polymerization tube, add dimethyl sulfoxide (DMSO) to dissolve, and add 0.3% (molar ratio) of azobisisobutyronitrile (AIBN). Put the polymerization tube into liquid nitrogen to cool it, carry out nitrogen gas-evacuation three times, seal the tube with an alcohol torch and put it in an oil bath at 70°C for 40 hours to react. After the reaction, the polymerization tube was opened to expose to the air. The DMSO solution of the polymer was precipitated three times in tetrahydrofuran to remove unreacted monomer.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com