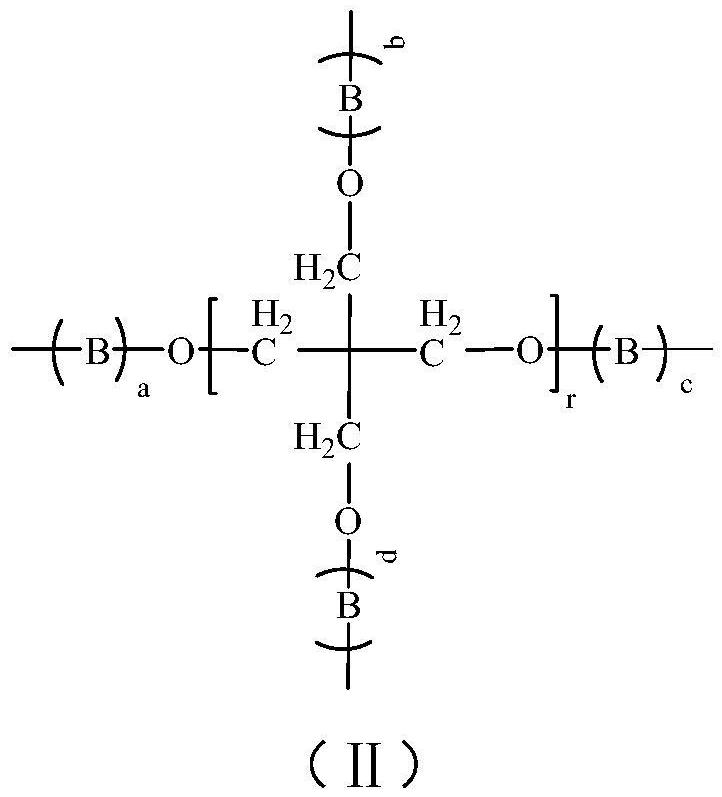
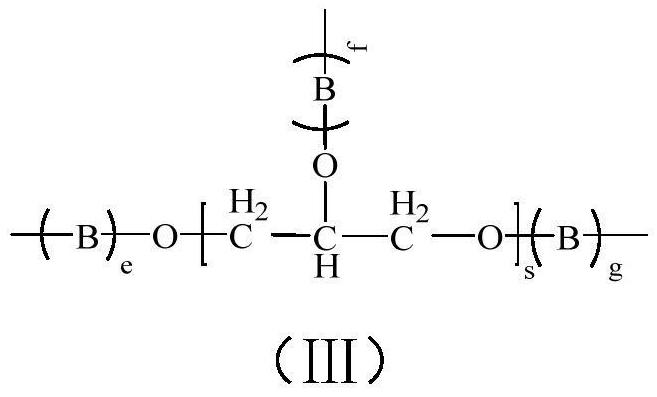
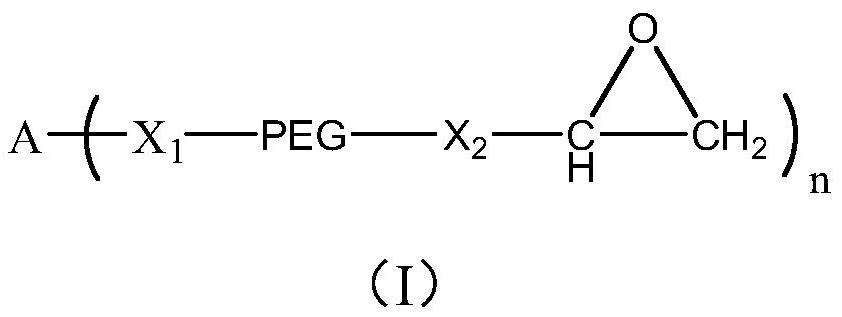Branched Polyethylene Glycol Epoxy Derivatives Crosslinked Sodium Hyaluronate Gel and Its Preparation and Application
An alcohol-epoxy, branched technology, applied in medical preparations containing active ingredients, medical preparations without active ingredients, tissue regeneration, etc., can solve the problems of difficult removal, hidden dangers of toxicity and cancer, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0151] Embodiment 1: Synthetic four-arm dodecaethylene glycol tetraglycidyl ether (Ia)
[0152] The four-arm dodecaethylene glycol tetraglycidyl ether of the following structure is synthesized:
[0153]
[0154] Add four-arm dodecaethylene glycol ( 0.1mol), tetrahydrofuran (THF, 100mL) and potassium hydroxide (0.8mol), stirred in a water bath, then dripped epichlorohydrin (ECH, 1.2mol) into the reaction system, controlled the reaction temperature not to exceed 35°C, and reacted at room temperature overnight. After the reaction, the reaction solution was filtered, and the filter residue was washed with dichloromethane, then the filtrate was collected, and the dichloromethane was removed by rotary evaporation to obtain a crude product. The crude product was separated by a silica gel column to obtain pure tetraethylene glycol diglycidyl ether.
[0155] 1 H-NMR (DMSO-d 6 ): 2.26-2.30(m, 8H), 2.54-2.55(m, 4H), 2.72-2.73(m, 4H), 3.09-3.10(m, 4H), 3.17-3.28(m, 20H), 3.35-3.6...
Embodiment 2
[0157] Embodiment 2: Synthetic four-arm tetraglycidyl glycol tetraglycidyl ether (Ib)
[0158] Synthesize four-arm tetraglycidyl glycol tetraglycidyl ether with the following structure:
[0159]
[0160]To the three-neck flask, add four-arm tetracosanthylene glycol ( 0.1mol), tetrahydrofuran (THF, 100mL) and potassium hydroxide (0.8mol), stirred in a water bath, and then dripped epichlorohydrin (ECH, 1.2mol) into the reaction system, controlling the reaction temperature not to exceed 35°C, and reacting at room temperature overnight. After the reaction, the reaction solution was filtered, and the filter residue was washed with dichloromethane, then the filtrate was collected, and the dichloromethane was removed by rotary evaporation to obtain a crude product. The crude product was separated by a silica gel column to obtain pure tetraglycidyl ether.
[0161] 1 H-NMR (DMSO-d 6 ): 2.26-2.30(m, 8H), 2.54-2.55(m, 4H), 2.72-2.73(m, 4H), 3.09-3.10(m, 4H), 3.17-3.28(m, 20H), 3...
Embodiment 3
[0163] Embodiment 3: Synthesis of eight-arm tetraethylene glycol octaglycidyl ether (Ic)
[0164] Synthesize eight-arm tetraethylene glycol octaglycidyl ether of the following structure:
[0165]
[0166] Add octaarm tetraethylene glycol ( 0.1mol), tetrahydrofuran (THF, 100mL) and potassium hydroxide (1.6mol), stirred in a water bath, and then dripped epichlorohydrin (ECH, 2.4mol) into the reaction system, controlling the reaction temperature not to exceed 35°C, and reacting at room temperature overnight. After the reaction, the reaction solution was filtered, and the filter residue was washed with dichloromethane, then the filtrate was collected, and the dichloromethane was removed by rotary evaporation to obtain a crude product. The crude product was separated by silica gel column to obtain pure eight-arm tetraethylene glycol octaglycidyl ether.
[0167] 1 H-NMR (DMSO-d 6 ): 2.27-2.33(m, 16H), 2.54-2.55(m, 8H), 2.72-2.73(m, 8H), 3.09-3.10(m, 8H), 3.16-3.26(m, 24H), ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com