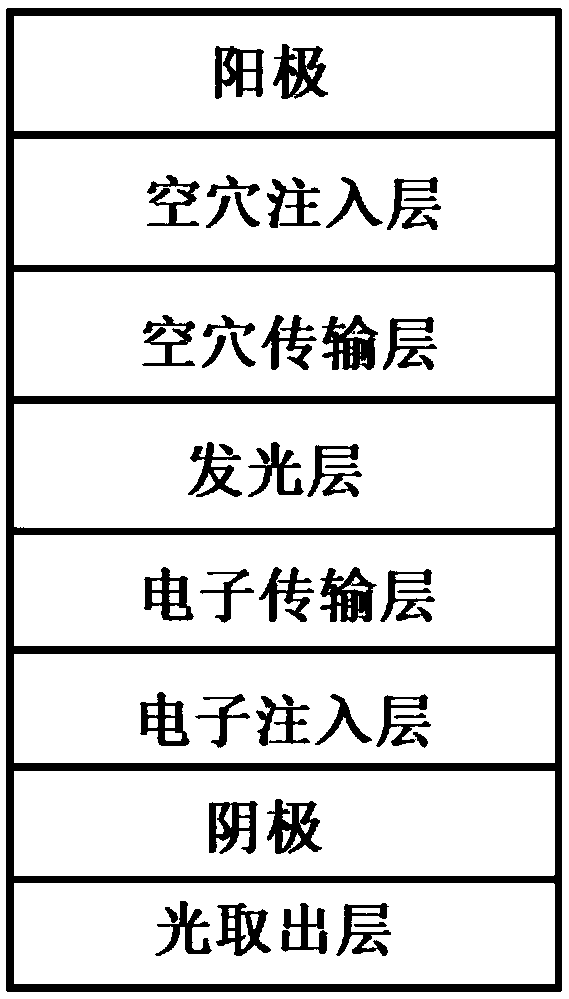
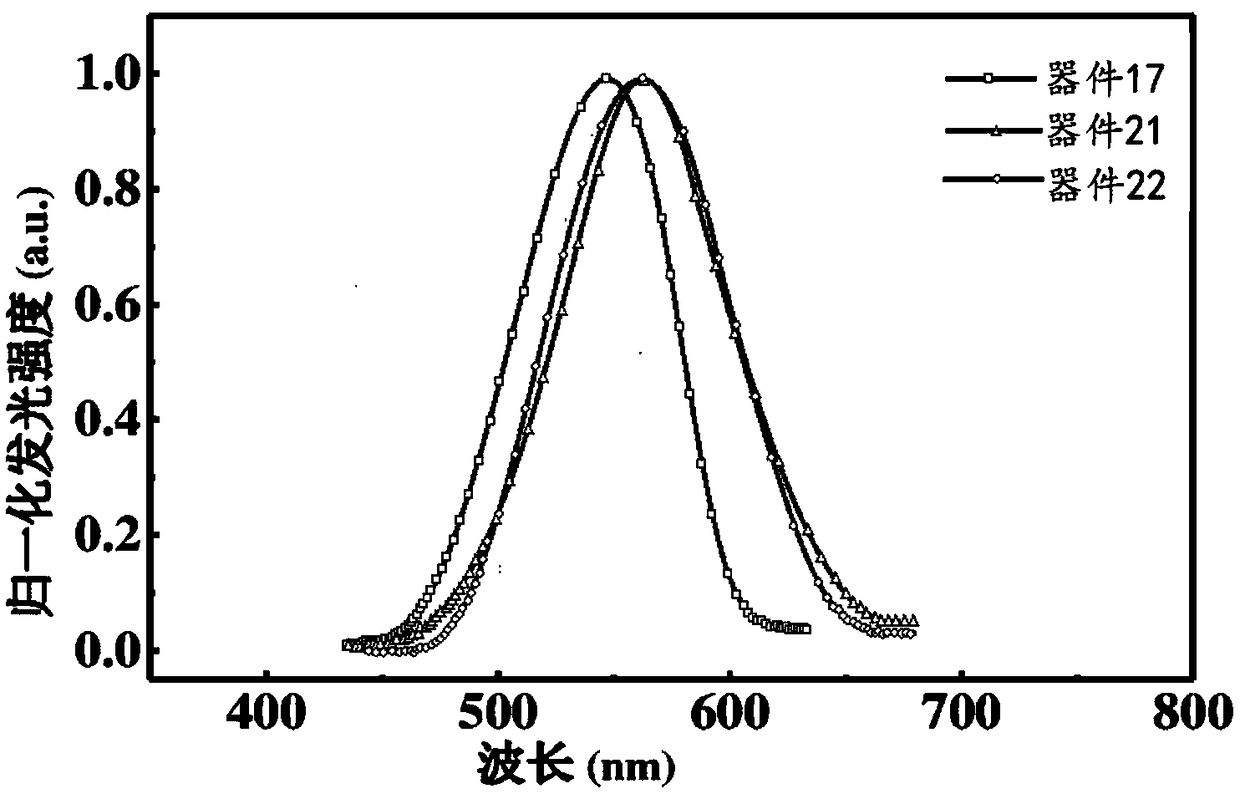
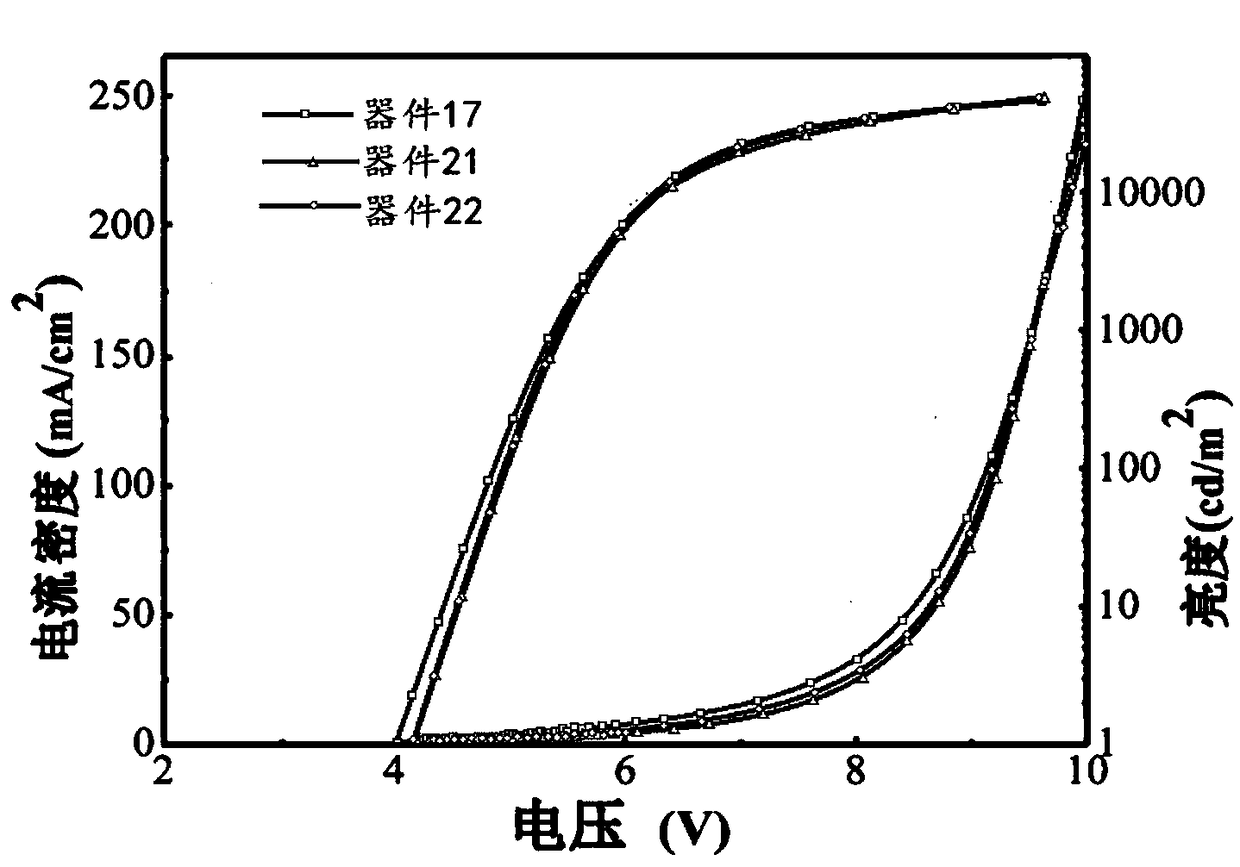
Light take-out layer material and application thereof
A light extraction layer, unsubstituted technology, applied in luminescent materials, electrical components, circuits, etc., can solve problems such as complex manufacturing processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: Compound 6 can be synthesized by the following method:
[0043]
[0044] (1) In a 500mL three-necked flask, add anthracen-1-ylboronic acid (13.32g, 60mmol), 4-bromophenylboronic acid (12.05g, 60mmol), potassium carbonate (16.58g, 120mmol), toluene (150mL), water (75mL), ethanol (75mL), under the protection of nitrogen, continue to add tetrakis(triphenylphosphine)palladium (0.14g, 0.12mmol), heat up to 75°C, react for 8h, HPLC monitors the completion of the reaction, and cool down to stop the reaction. Filtrate, concentrate the organic layer, mix with the filter residue and beat once with ethanol:ethyl acetate at a volume ratio of 10:1 to obtain 15.03 g of 3-(anthracen-1-yl)phenylboronic acid with a yield of 84%;
[0045] (2) In a 500mL three-necked flask, add 3-(anthracene-1-yl)phenylboronic acid (8.94g, 30mmol), 2,5-dibromo-1,3,4-thiadiazole (8.78g, 36mmol) , potassium carbonate (8.29g, 60mmol), toluene (100mL), water (50mL), ethanol (50mL), under nit...
Embodiment 2
[0048] Embodiment 2: Compound 19 can be synthesized by the following method:
[0049]
[0050] (1) Replace anthracene-1-ylboronic acid (13.32g, 60mmol) in Example 1(1) with 3-bromo-N, N-diphenylaniline (17.35g, 60mmol), 4-bromophenylboronic acid (12.05g, 60mmol) was replaced by 3-bromophenylboronic acid (12.05g, 60mmol), and the other steps were the same as in Example 1 (1), to obtain (3'-diphenylamino-[1,1'-linked Benzene]-3-yl)boronic acid 17.53g, yield 80%;
[0051] (2) Replace 3-(anthracene-1-yl)phenylboronic acid (8.94g, 30mmol) in Example 1(2) with (3'-diphenylamine-[1,1'-biphenyl]- 3-yl)boronic acid (10.96g, 30mmol), other steps are the same as in Example 1(2), to obtain 3'-(5-bromo-1,3,4-thiadiazol-2-yl)-N, N-diphenyl-[1,1'-biphenyl]-3-amine 10.75g, yield 74%;
[0052] (3) Replace 2-(3-(anthracene-1-yl)phenyl)-5-bromo-1,3,4-thiadiazole (6.26g, 15mmol) in Example 1(3) with 3 '-(5-bromo-1,3,4-thiadiazol-2-yl)-N,N-diphenyl-[1,1'-biphenyl]-3-amine (7.27g, 15mmol), ...
Embodiment 3
[0054] Embodiment 3: Compound 32 can be synthesized by the following method:
[0055]
[0056] (1) Anthracene-1-ylboronic acid (13.32g, 60mmol) in Example 1(1) is replaced by phenanthrene-4-ylboronic acid (13.32g, 60mmol), and other steps are the same as in Example 1(1), namely 14.67 g of (4-(phenanthren-4-yl)phenyl)boronic acid can be obtained with a yield of 82%;
[0057] (2) In a 250mL three-necked flask, add 2,5-dibromo-1,3,4-thiadiazole (3.66g, 15mmol), (4-(phenanthrene-4-yl)phenyl)boronic acid (10.73g , 36mmol), potassium carbonate (4.15g, 30mmol), toluene (50mL), water (25mL), ethanol (25mL), under nitrogen protection, continue to add tetrakis (triphenylphosphine) palladium (0.04g, 0.03mmol), The temperature was raised to 100° C., and the reaction was carried out for 18 hours. The completion of the reaction was monitored by HPLC, and the reaction was stopped when the temperature was lowered to room temperature. Extracted with dichloromethane, separated, concentrate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com