Anti-human-IgG monoclonal antibody, hybridoma cell strain capable of secreting anti-human-IgG monoclonal antibody and application of anti-human-IgG monoclonal antibody
A technology of hybridoma cell lines and monoclonal antibodies, applied in biochemical equipment and methods, instruments, microorganisms, etc., can solve problems such as low accuracy rate, inapplicability to grassroots promotion, and errors in naked eye observation, and achieve loose storage conditions and operations conditions, excellent long-term and thermal stability, and the effect of good specific binding ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] The immunization of embodiment 1 mice
[0062] Human blood-derived IgG antigen (Sichuan Mike Bio-New Material Technology Co., Ltd., batch number 091416) was diluted to 3 mg / ml with normal saline, and mixed with an equal volume of Freund's complete adjuvant (Sigma Company, product number SLBF-9338V) (100 μg / piece BALB / c mouse), be emulsified into oily emulsion with 1ml syringe, stop emulsification until the oily emulsion dripped into water does not disperse, and this emulsion is subcutaneously administered to BALB / c mouse (Chengdu Dashuo Experimental Animal Center, 5-week-old female, 3) Immunization was enhanced 14 days after the first immunization, human IgG was mixed with an equal volume of Freund's incomplete adjuvant (Sigma Company, product number SLBM9367V) (50 μg / body BALB / c After emulsification of mice), the immunization dose was 100 μl / mouse, and the immunization was boosted once every other week, and the tail blood was collected before each immunization, and the...
Embodiment 2
[0065] The preparation of embodiment 2 hybridoma cell lines
[0066] 2-1 Preparation of feeder cells
[0067] Peritoneal macrophages of normal 12-week-old BALB / c mice were used as feeder cells. One day before the fusion, BALB / c was sacrificed by taking blood from the eyes and pulling the neck, soaking in 0.1% bromogeramine for 1 minute, then soaking in 75% alcohol for 1 minute, lifting the abdominal skin from the hind abdomen with sterile scissors in an ultra-clean bench to expose the peritoneum . Wipe the peritoneum with an alcohol swab to disinfect. Inject 2ml of RPMI1640 culture solution into the peritoneal cavity with a syringe, taking care to avoid penetrating into the intestine. Fix the syringe with the right hand so that the needle remains in the abdominal cavity, and gently massage the abdomen with the alcohol cotton ball in the left hand for 1 minute, and then suck out the injected culture solution. Centrifuge at 1000r / min for 5-10 minutes, discard the supernatant...
Embodiment 3
[0080] The preparation of embodiment 3 monoclonal antibody
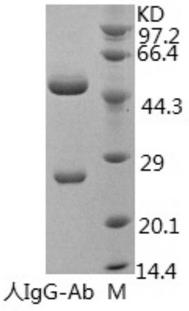
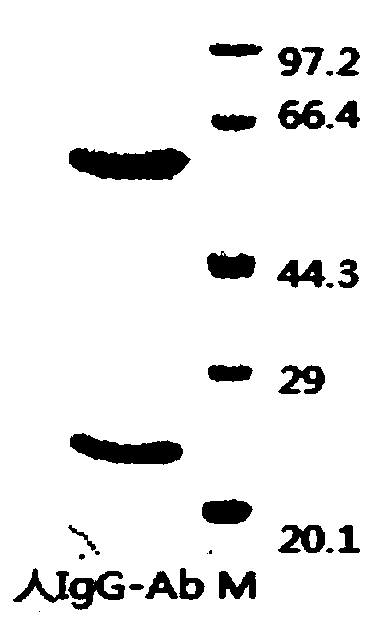
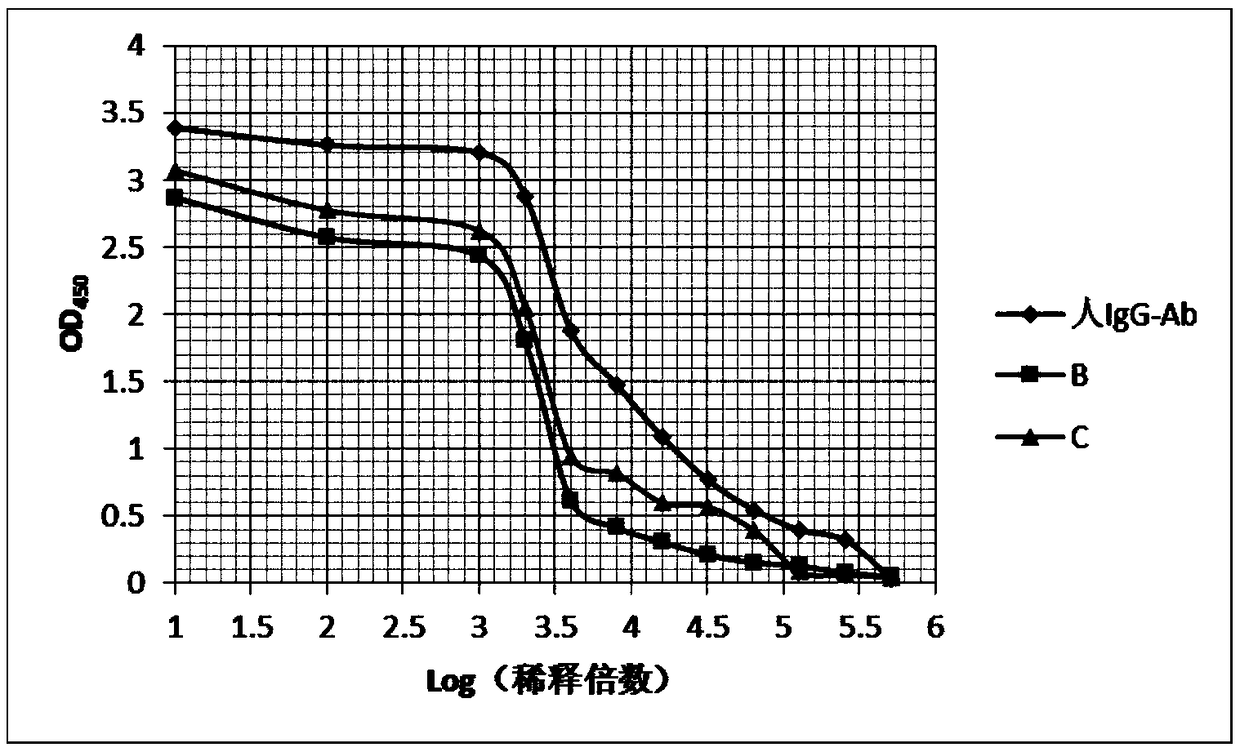
[0081] Select healthy BALB / c mice of 12-14 weeks, inject 0.5mL liquid paraffin (Tianjin Kemiou) intraperitoneally into each mouse, and inject 2×10 6 a hybridoma cell. Ascites can be produced 7-10 days after cell inoculation. Observe the occurrence of ascites in mice every day. If the abdomen is obviously enlarged and the skin feels tense when touched with hands, the mice can be killed by pulling the neck, and the ascites can be sucked into the test tube with a dropper. One mouse can obtain 1-5mL ascites. The collected ascites was centrifuged to obtain the supernatant, and a small sample was taken and stored in a -20°C refrigerator. The ascitic fluid was saturated and precipitated with ammonium sulfate, and then purified by protein A affinity chromatography. The purity of the antibody (the antibody of the present invention is referred to as human IgG-Ab) detected by SDS-PAGE was greater than 90%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com