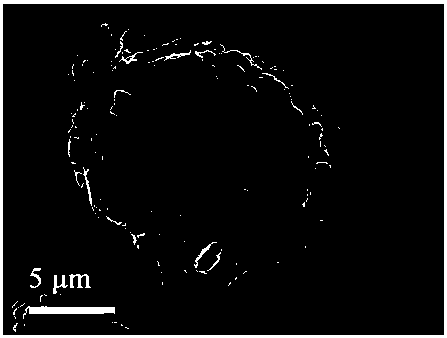
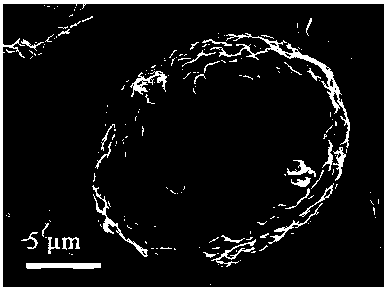
A method for preparing a lithium-rich manganese-based cathode material coated with lithium titanium phosphate
A lithium-rich manganese-based, cathode material technology, applied in the direction of positive electrode, battery electrode, active material electrode, etc., can solve the problems of electrochemical performance to be improved, uncontrollable hydrothermal method, unsuitable for large-scale production, etc. The effect of alleviating layered-spinel phase transition, excellent cycle stability, and improving poor electrical conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] This embodiment includes the following steps:
[0045] (1) Preparation of lithium-rich manganese-based cathode materials
[0046] Weigh 0.0840mol lithium-rich manganese-based precursor Mn 4 / 6 Ni 1 / 6 co 1 / 6 CO 3 With 0.1302molLiOH. h 2O was hand-milled and mixed (the amount of excess lithium was 5%), and the grinding time was 2h; the raw materials were put into a crucible, placed in a muffle furnace, and pre-sintered at 500°C for 6h in an air atmosphere, and then sintered at 900°C for 10h, and the temperature was raised. The rate is 5°C / min. After the furnace temperature is cooled to room temperature, the lithium-rich manganese-based cathode material 0.5Li 2 MnO 3 0.5Li(Ni 1 / 3 co 1 / 3 mn 1 / 3 )O 2 ;
[0047] (2) Dissolve 3.0000 g of lithium-rich manganese-based cathode material (337.8 mmol) obtained in step (1) in 90 mL CH 3 CH 2 OH, stir evenly, add 20.0000mmol C 16 h 36 o 4 Ti (density 0.9660g / cm 3 ), and stir evenly to obtain a black suspension a;
[...
Embodiment 2
[0054] This embodiment includes the following steps:
[0055] (1) Preparation of lithium-rich manganese-based cathode materials
[0056] Weigh 0.1680mol lithium-rich manganese-based precursor Mn 4 / 6 Ni 1 / 6 co 1 / 6 CO 3 with 0.1310molLi 2 CO 3 Carry out hand grinding and mixing (the amount of lithium is 6%), and the grinding time is 3 hours; put the raw materials into a crucible, place them in a muffle furnace, and pre-sinter at 500°C for 6 hours in an air atmosphere, then sinter at 950°C for 15 hours, and then heat up The rate is 5°C / min. After the furnace temperature is cooled to room temperature, the lithium-rich manganese-based cathode material 0.5Li 2 MnO 3 0.5Li(Ni 1 / 3 co 1 / 3 mn 1 / 3 )O 2 ;
[0057] (2) Dissolve 1.5000 g (168.9 mmol) of the lithium-rich manganese-based cathode material obtained in step (1) in 90 mL CH 3 CH 2 OH, stir evenly, add 20.0000mmol C 16 h 36 o 4 Ti (density 0.9660g / cm 3 ), and stir evenly to obtain a black suspension a;
[0058] ...
Embodiment 3
[0064] This embodiment includes the following steps:
[0065] (1) Preparation of lithium-rich manganese-based cathode materials
[0066] Weigh 0.1680mol lithium-rich manganese-based precursor Mn 4 / 6 Ni 1 / 6 co 1 / 6 CO 3 with 0.1302molLi 2 CO 3 Carry out hand-grinding and mixing (the amount of excess lithium is 5%), and the grinding time is 2 hours; put the raw materials into a crucible, place them in a muffle furnace, pre-sinter at 500°C for 5 hours, and then sinter at 900°C for 12 hours, with a heating rate of 3°C / min, after the furnace temperature is cooled to room temperature, the lithium-rich manganese-based cathode material 0.5Li 2 MnO 3 0.5Li(Ni 1 / 3 co 1 / 3 mn 1 / 3 )O 2 ;
[0067] (2) Dissolve 3.0000 g of lithium-rich manganese-based cathode material (337.8 mmol) obtained in step (1) in 40 mL CH 3 CH 2 OH, stir evenly, add 20.0000mmol C 16 h 36 o 4 Ti (density 0.9660g / cm 3 ), and stir evenly to obtain a black suspension a;
[0068] (3) Add 30.0000mmolH 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com