Method for synthesizing 3,4-dihydroisoquinoline by semi-dehydrogenation oxidation of 1,2,3,4-tetrahydroisoquinoline
A technology of tetrahydroisoquinoline and dihydroisoquinoline, which is applied in chemical instruments and methods, organic chemistry, chemical/physical processes, etc., can solve the problems of high synthesis cost, limited production, etc., and achieves high selectivity and preparation. The method is simple and easy to implement, and it is beneficial to the effect of large-scale industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0017] (1) [(NH 4 ) 2 MoS 4 ] Preparation: 7.5g (NH 4 ) 6 Mo 7 o 24 • 4H 2 Add O into 50ml of concentrated ammonia water, stir until dissolved, then add 50ml of ammonium sulfide solution with a mass fraction of 20%, keep the temperature at 80°C for 1 hour, cool the reaction system to 0°C, wash with water and ethanol, and dry After obtaining the compound [(NH 4 ) 2 MoS 4 ];
[0018] (2) MoS 2 / ZnIn 2 S 4 Preparation: 200mg ZnIn 2 S 4 Solid powder and [(NH 4 ) 2 MoS 4 ] into a dry schlenk tube and replace with N 2 Wash the air three times, then add 3ml of methanol solution to remove oxygen, and then use a 300W xenon lamp to illuminate under visible light for 3h, after washing and drying, dark yellow solid powder MoS 2 / ZnIn 2 S 4 , where MoS 2 The loading amount is 1.0 wt%.
Embodiment 1
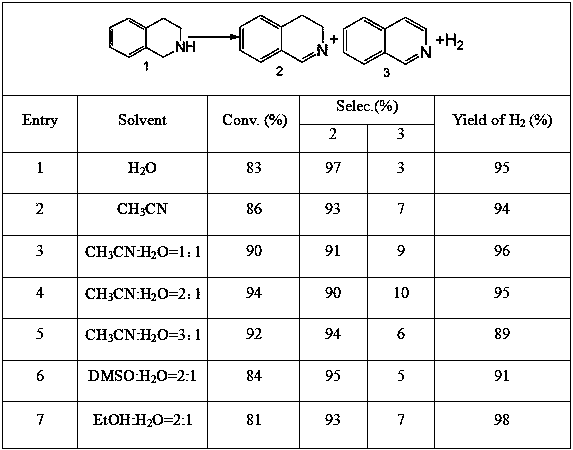
[0020] With 1,2,3,4-tetrahydroisoquinoline as substrate, MoS 2 / ZnIn 2 S 4 as a photocatalyst, in N 2 Atmosphere, with acetonitrile (CH 3 CN), water (H 2O), a mixed solution of acetonitrile and water, a mixed solution of DMSO and water, and a mixed solution of EtOH and water were used as solvents, and were illuminated for 12 hours under visible light. The reaction results are shown in the table below, where when the volume ratio of acetonitrile to water is 2:1, the conversion rate and selectivity are higher than other solvents as a whole.
[0021] Table 1 Reaction solvent to MoS 2 / ZnIn 2 S 4 Influence of photocatalytic semidehydrogenation performance of 1,2,3,4-tetrahydroisoquinoline
[0022]
[0023] Note: The solvent ratios in Table 1 are volume ratios.
Embodiment 2
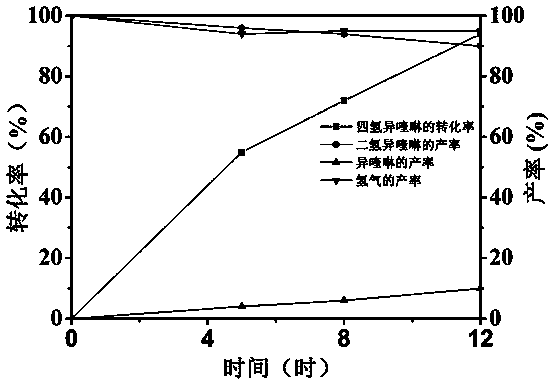
[0025] With 1,2,3,4-tetrahydroisoquinoline as substrate, MoS 2 / ZnIn 2 S 4 as a photocatalyst, in N 2 Under the atmosphere, using the mixed solution of acetonitrile and water with a volume ratio of 2:1 as the solvent, the trend chart of the change of different products over time was made. The result of the reaction is as figure 1 shown by figure 1 It can be seen that with the increase of time, the conversion rate of the substrate 1,2,3,4-tetrahydroisoquinoline is constantly increasing, and the conversion rate can reach 94% after 12 hours of reaction, and the yield of the main product 3.4-dihydroisoquinoline Decrease slowly with the increase of reaction time, and the productive rate reaches 90% after 12 hours of reaction, and the productive rate of by-product isoquinoline increases slowly with the increase of reaction time, and the productive rate reaches 10% after 12 hours of reaction, shows that with the prolongation of time, A small amount of 3,4-dihydroisoquinoline con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com