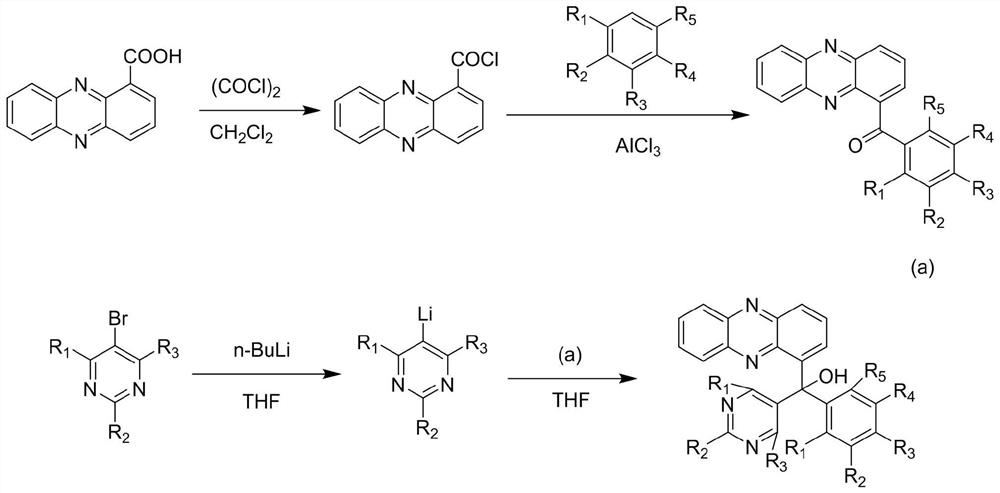
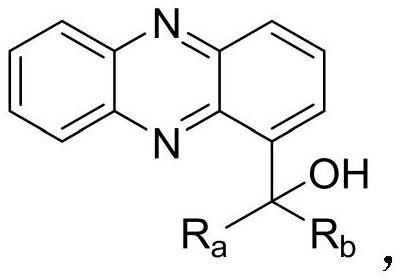
1-phenazinyl (phenyl) (5-pyrimidinyl) methanol compound and its preparation method and application
A compound and phenazine-based technology, applied in the field of 1-phenazine-based methanol compounds and their preparation, can solve the problems of poor solubility and difficult formulation processing, and achieve good antifungal effect, good solubility, and good bactericidal activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 Synthesis of 1-phenazinyl(phenyl)(5-pyrimidinyl)methanol
[0047] 1. Synthesis of phenazinyl-1-formyl chloride:
[0048]
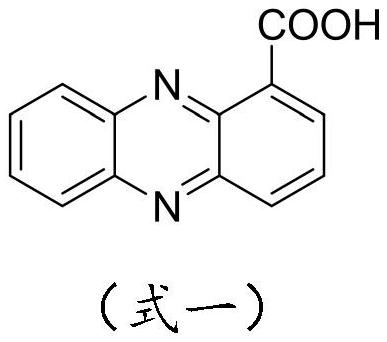
[0049] Add 2.5 g (11.2 mmol) of phenazinyl-1-carboxylic acid, 30 ml of dichloromethane, drop 1 to 2 drops of DMF into a 100 ml single-neck reaction flask, slowly add 3.0 g of oxalyl chloride (to prevent flooding), then heat to reflux The reaction was carried out until the solid of suzinomycin completely disappeared, and the reflux reaction was continued for 2 to 3 hours. The solvent was dried on a rotary evaporator, and a small amount of dichloromethane was added to dissolve it. Then spin to dry, and take away the excess oxalyl chloride as much as possible. for use in the next step.
[0050] 2. Synthesis of phenazine-1-yl (phenyl) ketone:
[0051]
[0052] Add 30 ml of anhydrous benzene solution to the single-necked bottle containing phenazine-1-formyl chloride, slowly add 8.4 grams (22.4 mmol) of anhydrous AlCl 3 , the reaction w...
Embodiment 2
[0061] Example 2: Synthesis of phenazin-1-yl(4-phenoxy)(5-pyrimidinyl)methanol:
[0062] 1. Synthesis of phenazinyl-1-formyl chloride:
[0063]
[0064] Add 2.5 g (11.2 mmol) of phenazinyl-1-carboxylic acid, 30 ml of dichloromethane, drop 1 to 2 drops of DMF into a 100 ml single-neck reaction flask, slowly add 3.0 g of oxalyl chloride (to prevent flooding), then heat to reflux The reaction was carried out until the solid of suzinomycin completely disappeared, and the reflux reaction was continued for 2 to 3 hours. The solvent was dried on a rotary evaporator, and a small amount of dichloromethane was added to dissolve it. Then spin to dry, and take away the excess oxalyl chloride as much as possible. for use in the next step.
[0065] 2. Synthesis of phenazine-1-yl(4-phenoxyphenyl)methanone:
[0066]
[0067] Add 30 ml of anhydrous diphenyl ether solution to the single-necked bottle containing phenazine-1-formyl chloride, slowly add 3.03 g (22.6 mmol) of anhydrous AlCl...
Embodiment 3
[0076] Example 3: Synthesis of (2,3-dimethylphenyl)(phenazin-1-yl)(5-pyrimidinyl)methanol
[0077] 1. Synthesis of phenazinyl-1-formyl chloride:
[0078]
[0079] Add 2.5 g (11.2 mmol) of phenazinyl-1-carboxylic acid, 30 ml of dichloromethane, drop 1 to 2 drops of DMF into a 100 ml single-neck reaction flask, slowly add 3.0 g of oxalyl chloride (to prevent flooding), then heat to reflux The reaction was carried out until the solid of suzinomycin completely disappeared, and the reflux reaction was continued for 2 to 3 hours. The solvent was dried on a rotary evaporator, and a small amount of dichloromethane was added to dissolve it. Then spin to dry, and take away the excess oxalyl chloride as much as possible. for use in the next step.
[0080] 2. Synthesis of (2,3-dimethylphenyl)(phenazine-1-yl)methanone:
[0081]
[0082] Add 30 ml of anhydrous o-xylene solution to the single-necked bottle containing phenazine-1-formyl chloride, slowly add 3.0 g (22.6 mmol) of anhydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com